Toxicity Studies of Abutilon crispum and Indigofera prostrata Whole Plants on Wistar Rats
Main Article Content
Abstract
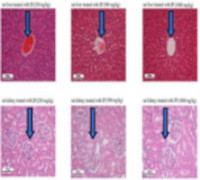
Abutilon crispum and Indigofera prostrata are widely distributed Indian medicinal plants. They are extensively used to treat various diseases by Indian herbal medicine practitioners. Although, preclinical studies have been reported on these plants, the existing toxicological data is inadequate. This study aims to evaluate the acute and chronic toxicity of Abutilon crispum and Indigofera prostrata whole plants in rats. Extracts of the plants were obtained by maceration in methanol. For the acute toxicity study, a single oral dose of 2000 mg/kg of the methanol extracts of the plants were administered to the rats, and the rats were observed for 14 days for signs of toxicity or death. For the chronic toxicity study, the rats were administered 250, 500, and 1000 mg/kg of the extracts once daily for 90 days. Throughout the treatment period, the rats were observed for signs of toxicity, mortality and body weight changes. After 90 days, the animals were sacrificed and blood samples were collected for haematological and biochemical analysis, and vital organs (liver and kidneys) were used for histological examination. Acute toxicity study revealed that the methanol extracts of Indigofera prostrata and Abutilon crispum were non-toxic up to a dose of 2000 mg/kg. Similarly, in the chronic toxicity study, no significant changes were observed in the body weights, haematological and biochemical parameters of the rats. The histoarchitecture of the liver and kidneys appeared normal. Therefore, the extracts of Abutilon crispum and Indigofera prostrata whole plants are non-toxic, and is relative safe on chronic administration.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
Arome D and Chinedu E. The importance of toxicity testing. J Pharm BioSci. 2013; 4:146-148.
Gandhare B, Kavimani S, Rajkapoor B. Acute and subacute toxicity study of methanolic extract of Ceiba pentandra (Linn.) Gaertn. on rats. J Sci Res. 2013; 5(2):315-324. DOI: https://doi.org/10.3329/jsr.v5i2.11800
Saleem U, Amin S, Ahmad B, Azeem H, Anwar F, Mary S. Acute oral toxicity evaluation of aqueous ethanolic extract of Saccharum munja Roxb. roots in albino mice as per OCED 425 TG. Toxicol Rep. 2017; 4:580-585. DOI: https://doi.org/10.1016/j.toxrep.2017.10.005
Charles LP and Bhaskar RK. Acute and sub-chronic toxicological studies on methanolic stem extract of Acalypha indica Linn in albino wistar rats. Int J Pharm Pharm Sci. 2014; 6(9):560-563.
Vilash V, Suja SR, Latha PG, Shine VJ, Rajasekhran S. Chronic oral toxicity studies of crude ethanolic extract and ethanolic fraction of Pellionia heyneana wedd. leaf in Wistar rats. Int J Pharm Pharm Sci. 2016; 8(8):306-312. DOI: https://doi.org/10.5530/pj.2016.6.6
Soon YY and Tan BK, Evaluation of the hypoglycemic and anti-oxidant activities of Morinda officinalis in streptozotocin-induced diabetic rats. Singapore Med J 2002; 43(2):077-085.
Trinder P. Determination of glucose-by-glucose oxidase method. Ann Clin Biochem. 1969; 6:24-26. DOI: https://doi.org/10.1177/000456326900600108
Malloy HT and Evelyn KA. The determination of bilirubin with the photoelectric colorimeter. J Biochem. 1937; 119:481-490. DOI: https://doi.org/10.1016/S0021-9258(18)74392-5
Raja S and Ravindranadh K. Acute and subchronic toxicity studies of Limnophila heterophylla and Michelia champaca. Int J Res Pharm Pharmacother. 2017; 6(3):348-357.
Eran BA, Noah S, Lee HG, Kamer M, Suha O, Elad S. Potential risks associated with traditional herbal medicine use in cancer care: A study of middle eastern oncology health care professionals. Cancer. 2016; 122:598-610. DOI: https://doi.org/10.1002/cncr.29796
Kwan YP, Ibrahim D, Yeng C, Subramaniam S, Sreenivasan S. Acute and subchronic toxicity study of Euphorbia hirta L. methanol extract in rats. Hindawi Publishing Corporation; Biomed Res. Int. 2013; 1-14 p. DOI: https://doi.org/10.1155/2013/182064
Yu J, Wang Y, Qian H, Zhao Y, Liu B, Fu C. Polyprenols from Taxus chinensis var. mairei prevent the development of ccl4-induced liver fibrosis in rats. J Ethnopharmacol. 2012; 142(1):151-160. DOI: https://doi.org/10.1016/j.jep.2012.04.030
Krishna RG and Sundararajan R. Toxicity studies of Bougainvillea glabra and Mucuna pruriens. Int J Pharm Sci Res. 2020; 11(10):1000-1008. DOI: https://doi.org/10.26452/ijrps.v11i1.1898
Zhiqiang J, Min L, Zhe Q, Yuanyuan Z, Shutong Y, Anshan S. Toxic effects of zearalenone on oxidative stress, inflammatory cytokines, biochemical and pathological changes induced by this toxin in the kidney of pregnant rats. Environ Toxicol Pharmacol. 2014; 37(2):580-591. DOI: https://doi.org/10.1016/j.etap.2014.01.010
Idakwoji PA, Oguche M, Sule FA, Oniwon WO, Shaibu IE, Edegbo E, Onoja AO, Ukwubile II. In vitro antidiabetic activity and sub-chronic toxicity profile of ethanol extract of Tephrosia bracteolata leaves. Trop J Nat Prod Res. 2024; 8(4): 012-7019. DOI: https://doi.org/10.26538/tjnpr/v8i4.37
Onyeyilli PA, Iwuoha CL, Akinniya JA. Chronic toxicity study of Ficus platyphtlla blume in rats. West Afr J Pharmacol Drug Res. 1998; 14:27-30. DOI: https://doi.org/10.4314/wajpdr.v14i1.53414
Thomas A and Radhakrishnan EK. Acute and subacute oral toxicity studies of hydroalcoholic extract of Terminalia arjuna (Roxb.) bark in rodents. Trop J Nat Prod Res. 2023; 7(7):3351-3359 DOI: https://doi.org/10.26538/tjnpr/v7i7.11