Molecular Docking, Contraceptive Property and Histopathological Changes in Experimental Models by Digitaria exilis Grain Extract via Interference with Steroidogenesis at Ovarian Level
Main Article Content
Abstract
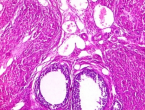
The use of Digitaria exilis in traditional medicine for contraception has been scientifically validated without a report on the mechanism of its possible risk to women's fertility. Therefore, this study evaluated the potential mechanism of contraceptive property of aqueous grain extract of Digitaria exilis (AGEDE) in female Wistar rats using molecular docking and histopathological analysis. Twenty female rats were grouped into four (n=5). Group A served as control while groups B-D received (50, 100, and 200 mg/kg BW) of AGEDE for fourteen days. Serum and ovary levels of some reproductive biomarkers and histopathology and molecular docking analysis were conducted. Progesterone, FSH, LH, estrogen, and prolactin levels were altered at different AGEDE dosal levels in the serum and ovary of the animals. The histoarchitecture of ovarian and uterine tissues was altered by AGEDE, with severe infiltration of inflammatory cells, degenerated luteal cells, loosed ovarian stroma with severe vascular congestion, as well as degenerated endometrial glands with distorted hyperplasia, endometrium layer with deranged vascularization and severely fibrotic endometrium, respectively. Phytochemical analyses of AGEDE identified flavonoids, saponins, and tannins. Molecular docking analysis of previously documented compounds with 3β-hydroxysteroid dehydrogenase (3βHSD) revealed that ethylacetate had the highest docking score (-4.69227982), followed closely by 2,5-dimethylhydrazine (-4.61382103), while pyrazine (-3.80633807) had the least binding affinity. AGEDE demonstrated toxicity to the female reproductive organs via alteration and/or interference with steroidogenesis at the molecular, hormonal, enzymic, uterus, and ovarian levels. These interferences may affect fertility, conception, and pregnancy. Therefore, Digitaria exilis may be explored as a fertility-controlling agent.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.Selemani M, Makundi RH, Massawe AW, Mhamphi G, Mulungu LS, Belmain SR. Impact of contraceptive hormones on the reproductive potential of male and female commensal black rats (Rattus rattus). Integr Zool. 2022; 17(6): 991-1001. doi: 10.1111/1749-4877.12563. DOI: https://doi.org/10.1111/1749-4877.12563
2.Teal S, Edelman A. Contraception Selection, Effectiveness, and Adverse Effects: A Review. JAMA. 2021; 326 (24): 2507–2518. doi:10.1001/jama.2021.21392. DOI: https://doi.org/10.1001/jama.2021.21392
3.Hilz EN, Olvera ME, Jun D, Chadha M, Gillette R, Monfils M, Gore AC, Lee HJ. Hormonal contraceptives alter amphetamine place preference and responsivity in the intact female rat. Behav. Neurosci. 2022; 136(4): 318–329. https://doi.org/10.1037/bne0000520. DOI: https://doi.org/10.1037/bne0000520
4.Santoso AW, Amalia E, Sari KI, Takarini V, Sufiawati I. Histopathological evaluation of wound healing and anti-inflammatory effects of Granola potato peel ethanol extract in rat oral mucosa. J Exp Pharmacol. 2024; 16: 377-395. https://doi.org/10.2147/JEP.S487373. DOI: https://doi.org/10.2147/JEP.S487373
5.Kondaiah G, Srineetha U, Kumar DVN, Narasimha Rao CN. Histopathological changes in the kidney of rats exposed to aluminum: a review. Int’l J Progr. Res Engr Mgmt. Sci. 2024; 4(8): 900-906. DOI: https://www.doi.org/10.58257/IJPREMS35829.
6.Chachulski L, Windshugel B. LEADS-FRAG: A Benchmark Data Set for Assessment of Fragment Docking Performance. J Chem Info Model. 2020; 60 (12): 6544–6554. doi:10.1021/acs.jcim.0c00693. DOI: https://doi.org/10.1021/acs.jcim.0c00693
7.Ballante F, Kooistra AJ, Kampen S, de-Graaf C, Carlsson J. Structure-Based Virtual Screening for Ligands of G Protein-Coupled Receptors: What Can Molecular Docking Do for You? Pharmacol Rev. 2021; 73 (4): 527–565. doi:10.1124/pharmrev.120.000246. DOI: https://doi.org/10.1124/pharmrev.120.000246
8.Morales-Payan JP, Richard OJ, Julio CT, Francisco TS. Digitaria exilis as a crop in the Dominican Republic. In: Janick, J., Whipkey, A. (Eds.), Supplement to: Trends in New Crops and New Uses. ASHS Press, Alexandria, VA. 2002.
9.Adoukonou-Sagbadja AH. Genetic characterization of traditional Fonio millets (Digitaria exilis, D. iburua Stapf) landraces from West Africa: implications for conservation and breeding. PhD thesis submitted by MSc Hubert Adoukonou-Sagbadja to Justus-Liebig University, Germany from University of Abomey-Calavi, Giessen, Cotonu, Benin Republic.pp.107 BP 526, 2010.
10.Heuze V, Tran G, Hassoun P, Lebas F. Fonio (Digitaria exilis) grain. Feedipedia, a programme by INRAE, CIRAD, AFZ and FAO. https://feedipedia.org/node/228. Last updated on August 30, 2019, 11:04.
11.Taylor JRN, Schober TJ, Bean SR. Novel food and non-food uses for sorghum and millets. J Cereal Sci. 2006; 44: 252–271. DOI: https://doi.org/10.1016/j.jcs.2006.06.009
12.Adams DM, Yakubu MT. Aqueous extract of Digitaria exilis grains ameliorate diabetes in streptozotocin-induced diabetic male Wistar rats. J Ethnopharmacol. 2020; 249: 112383. DOI: https://doi.org/10.1016/j.jep.2019.112383
13.Glew RH, Laabes EP, Presley JM, Schulze J, Andrews R, Wang Y, Chang Y, Chuang L. Fatty acid, amino acid, mineral and antioxidant contents of Acha (Digitaria exilis) grown on the Jos Plateau, Nigeria. Int’l J Nutr Metab. 2013; 5(1): 1–8.
14.Ballogou VY, Soumanou MM, Toukourou F, Hounhouigan JD. Structure and nutritional composition of Fonio (Digitaria exilis) grains: a review. Int’l Res J Biol Sci. 2013; 2(1): 73–79.
15.Alegbejo JO, Ameh DA, Ogala WM, Ibrahim S. Glycaemic index and load of Acha (Fonio) in healthy and diabetic subjects. J Pure Appl Microbiol. 2011; 5(1): 117–122.
16.Egwim EC, Oloyede OB. Immobilization of α-amylase from Acha (Digitaria exilis) on different cellulose fibre materials. Asian J Biochem. 2008; 3(3): 169–175. DOI: https://doi.org/10.3923/ajb.2008.169.175
17.Olagunju AI, Omoba OS, Enujiugha VN, Aluko RE. Development of value-added nutritious crackers with high antidiabetic properties from blends of Acha (Digitaria exilis) and blanched Pigeon pea (Cajanus cajan). Food Sci Nutr. 2018; 6: 1791–1802. DOI: https://doi.org/10.1002/fsn3.748
18.Adams MD, Muftaudeen TK, Saliu OA. Polyphenol-rich extract of Digitaria exilis (Kippist) grain lowers gastrointestinal dysmotility and enhanced colonic peristalsis in rifaximin-induced constipated rats. Nig J Biochem Mol Biol. 2023; 38(3): 131-138. https://dx.doi.org/10.4314/njbmb.v38i3.4. DOI: https://doi.org/10.4314/njbmb.v38i3.4
19.Babarinde GO, Ebun AA, Adegbola PI. Hepatotoxicity and biochemical evaluation of a novel breakfast food produced from the blend of white Fonio (Digitaria iburua/exilis Stapf) and pigeon pea (Cajanus cajan (L.) Millspaugh) in albino rats. Bull Nat Res Centre. 2020; 44:157. https://doi.org/10.1186/s42269-020-00409-6. DOI: https://doi.org/10.1186/s42269-020-00409-6
20.Aondoakaa IP, Arueya GL. Antihyperglycemic potentials of fermented Digitaria exilis polysaccharide partially substituted with Clendendrum volubile leaf extract. Food Chem Advan. 2024; 5:100844. https://doi.org/10.1016/j.focha.2024.100844. DOI: https://doi.org/10.1016/j.focha.2024.100844
21.Temple VJ, Bassa JD. Proximate chemical composition of Acha (Digitaria exilis) grain. J Sci Food Agric. 1991; 56: 561-563. DOI: 10.1002/jsfa.2740560415. DOI: https://doi.org/10.1002/jsfa.2740560415
22.Umerah NN, Asouzu AI. Modulation of the expression of lipogenic activity by malted hungry rice flour (Digitaria exilis) on rats induced with obesity. Asian J Res Biochem. 2023; 12(3): 30-40. 10.9734/ajrb/2023/v12i3238. DOI: https://doi.org/10.9734/ajrb/2023/v12i3238
23.Harborne JB. A guide to modern techniques of plant analysis. In: Phytochemical methods, Chapman and Hall Limited, London. 1973; 49-188.
24.Awe IS, Sodipo OA. Purification of saponins of root of Bhlighia sapida KOENIG-HOLL. Nig J Biochem. Mol. Biol. (Proceedings Supplement). 2001; 16: 201s-204s.
25.Odebiyi A, Sofowora AE. Phytochemical screening of Nigerian Medicinal Plant. Part III, Lloydia. 1978; 41:234-246.
26.Trease GE, Evans WC. Pharmacognosy. 11th (edn) Brailliar Tridel Can. Macmillian publishers, 1989.
27.Ganesan S, Bhatt RY. Qualitative Nature of Some Traditional Crude Drugs available in Commercial Markets of Mumbai, Maharashtra, India. Ethnobot Leaflets. 2008; 12: 348-360.
28.Edeoga HO, Okwu DE, Mbaebie BO. Phytochemical constituents of some Nigerian medicinal plants. Afr J Biotechnol. 2005; 4(7): 685-688. DOI: https://doi.org/10.5897/AJB2005.000-3127
29.El-Olemy MM, Al-Muhtadi FJ, Afifi AA. Experimental Phytochemistry. A Laboratory Manual Saudi Arabia. King Saud University Press Riyadh, 1994; 3-137.
30.Adams MD, Eze ED. Borassus aethiopum (Mart.) ethanol fruit extract reverses alloxan-treatment alterations in experimental animals. Mediterr. J Nutr Metab. 2022; 15: 429–445. DOI:10.3233/MNM-211589. DOI: https://doi.org/10.3233/MNM-211589
31.Agoreyo FO, Onwegbu VI. Quantitative evaluation of Serum Progesterone levels in the three trimesters of pregnancy in Albino rat. J Appl Sci Environ Manag. 2015; 19(1): 77-79. DOI: 10.4314/jasem.v19i1.10. DOI: https://doi.org/10.4314/jasem.v19i1.10
32.Kosasa TS. Measurement of Human Luteinizing Hormone. J Reprod Med. 1981; 26: 201 -206.
33.Randolph JF, Jr M, Sowers IV, Bondarenko SD, Harlow JL, Luborsky RJ. Little change in estradiol and follicle-stimulating hormone across the early men menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab. 2004; 89(4): 1555-1561. DOI: https://doi.org/10.1210/jc.2003-031183
34.Blair IA. Analysis of estrogens in serum and plasma from postmenopausal women: past present, and future. Steroids. 2010; 75(4-5): 297-306. doi: 10.1016/j.steroids.2010.01.012. Epub 2010 Jan 28.
35.Ohnami S, Nakata H, Eto S. Immunoradiometric Assay for Prolactin in Serum and Tissue. Radioisotopes. 1990; 39: 393-395. DOI: https://doi.org/10.3769/radioisotopes.39.9_393
36.Shivanandappa T, Venkatesh S. Colorimetric Assay for β-hydroxysteroid dehydrogenase. Anal Biochem. 1997; 254: 57–61. Article No. ab972406. DOI: https://doi.org/10.1006/abio.1997.2406
37.Akira H, Yuji M, Yasushi M, Tadashi I, Mokio D, Hiroshi M. Highly sensitive assay of HMG-CoA reductase activity by LC-ESI-MS/MS. J Lipid Res. 2007; 48(5): 1212 – 1220: 2007. DOI: https://doi.org/10.1194/jlr.D600049-JLR200
38.Li H, Dutkiewicz EP, Huang YC, Zhou HB, Hsu CC. Analytical methods for cholesterol quantification. J Food Drug Anal. 2019; 27(2): 375-386. https://doi.org/10.1016/j.jfda.2018.09.001. DOI: https://doi.org/10.1016/j.jfda.2018.09.001
39.Rocha-Pereira C, Silva V, Costa VM, Silva R, Garcia J, Goncalves-Monteiro S. Histological and toxicological evaluation, in rat, of a P-glycoprotein inducer and activator: 1-(propan-2-ylamino)-4-propoxy-9H-thioxanthen-9-one (TX5), EXCLI J. 2019; 18: 697–722.
40.Lasekan OO, Feijao-Teixeira JP. Aroma compounds of malted Acha (Digitaria exilis Stapf). J Food Qual. 2004; 27:153-161.https://doi.org/10.1111/j.1745- 4557.2004.tb00645.x. DOI: https://doi.org/10.1111/j.1745-4557.2004.tb00645.x
41.O'Boyle, NM, Banck M, James CA, Morley C, Vandermeersch T, Hutchison GR. Open Babel: An open chemical toolbox, J Cheminformat. 2011; 3: 1-14. DOI: https://doi.org/10.1186/1758-2946-3-33
42.Oyebamiji AK, Akintelu SA, Afolabi SO, Akintayo ET, Akintayo CO, Ebenezer O. Computer aided study on cyclic tetrapeptide based ligands as potential inhibitors of Proplasmepsin IV. Sci Rep. 2025; 15: 13865. https://doi.org/10.1038/s41598-025-96410-y. DOI: https://doi.org/10.1038/s41598-025-96410-y
43.Edache EI, Adedayo A, Dawi HA, Ugbe FA. Drug-like screening, molecular docking, molecular dynamics simulations, and binding free energies on the interaction of pyrazole derivatives as inhibitors of lysosomal storage disorders and anticancer activity. Discov. Chem. 2024; 1: 22. https://doi.org/10.1007/s44371-024-00025-7. DOI: https://doi.org/10.1007/s44371-024-00025-7
44.Edache EI, Uzairu A, Mamza PA, Shallangwa GA, Azam M, Min K. Methimazole and propylthiouracil design as a drug for anti-graves’ disease: Structural studies, Hirshfeld surface analysis, DFT calculations, molecular docking, molecular dynamics simulations, and design as a drug for anti-graves’ disease. J. Mol. Struct. 2023; 1289: 135913. https://doi.org/10.1016/j.molstruc.2023.135913. DOI: https://doi.org/10.1016/j.molstruc.2023.135913
45.Atsukwei D, Eze ED, Adams MD, Tende JA, Olaniyan OT, Danmallam L. Contraceptive Effect of Ethanolic Extract of Dioscorea villosa Tuber on Reproductive Hormones of Female Wistar Rats. Int’l J Biochem Res Rev. 2015; 5(2): 135-144, 2015. DOI: 10.9734/IJBcRR/2015/13433.
46.Aluko VA, Okere SO, Olugbodi JO, Adams MD. Rosmarinic Acid Restored Testicular Function and Steroidogenic Hormone Capacity in Wistar Rats Exposed to Perfluorooctanoic Acid Toxicity. Trop J Nat Prod Res. 2024; 8(8): 8106-8111. https://doi.org/10.26538/tjnpr/v8i8.27. DOI: https://doi.org/10.26538/tjnpr/v8i8.27
47.Libby AE, Solt CM, Jackman MR, Sherk VD, Foright RM, Johnson GC, Nguyen TT, Breit MJ, Hulett N, Rudolph MC, Roberson PA, Wellberg EA, Jambal P, Scalzo RL, Higgins J, Kumar TR, Wierman ME, Pan Z, Shankar K, Klemm DJ, Moreau KL, Kohrt WM, MacLean PS. Effects of follicle-stimulating hormone on energy balance and tissue metabolic health after loss of ovarian function. Am J Physiol: Endocrinol Metab. 2024; 326 (5): E626-E639. https://doi.org/10.1152/ajpendo.00400.2023. DOI: https://doi.org/10.1152/ajpendo.00400.2023
48.Onyebuchi OJ, Onwurah EI, NO, Ikechukwu SE. Generational impact of exposure to volatile organic compounds from automobile paint spray on reproductive hormonal response in Wistar rats from parents to their filial generation (F1). J Hazard Mater Adv. 2025; 18: 100675. https://doi.org/10.1016/j.hazadv.2025.100675. DOI: https://doi.org/10.1016/j.hazadv.2025.100675
49.Xie H, Quian T, Liu L, Sun R, Che W, Zhao M, Hou X, Pan H, Su Y, Li J, Dong X, Liu P. Effect of progestin on thyroid function in female Wistar rats. Front. Endocrinol. 2024; 15: 1362774. DOI: 10.3389/fendo.2024.1362774. DOI: https://doi.org/10.3389/fendo.2024.1362774
50.Htoon KZ, Phyu KP, San TT, Kyaing KO. Effect of Murdannia edulis Faden roots on serum prolactin level and mammary glands of lactating female albino rats. Int J Basic Clin Pharmacol. 2024; Mar: 13(2):198-202. DOI: https://dx.doi.org/10.18203/2319-2003.ijbcp20240375. DOI: https://doi.org/10.18203/2319-2003.ijbcp20240375
51.Aritonang TR, Rahayu S, Sirait LI, Br Karo, M, Simanjuntak TP, Natzir R, Sinrang AW, Massi MN, Hatta M, Kamelia E. The Role of FSH, LH, Estradiol and Progesterone Hormone on Estrus Cycle of Female Rats. Int’l J Sci: Bas Appl Res (IJSBAR). 2017; 35(1): 92–100.
52.Yazawa T, Watanabe Y, Yokohama Y, Imamichi Y, Hasegawa K, Nakajima KI, Kitano T, Ida T, Sato T, Islam MS, Umezawa A, Takahashi S, Kato Y, Jahan S, Kawabe JI. Evaluation of 3β-hydroxysteroid dehydrogenase activity using progesterone and androgen receptors-mediated transactivation. Front. Endocrinol. 2024; 15:1480722. doi: 10.3389/fendo.2024.1480722. DOI: https://doi.org/10.3389/fendo.2024.1480722
53.Boguslawski W, Sokolowski W. HMG-CoA reductase activity in the microsomal fraction from human
placenta in early and term pregnancy. Int’l J Biochem. 1984; 16(9): 1023- 1026. https://doi.org/10.1016/0020-711X(84)90120-4. DOI: https://doi.org/10.1016/0020-711X(84)90120-4
54.Atsukwei D, Eze ED, Adams MD, Seriki SA, Ukpabi CN. Hypolipidaemic Effect of Ethanol Leaf Extract of Moringa Oleifera Lam. in Experimentally induced Hypercholesterolemic Wistar Rats. Int’l J Nutr Food Sci. 2014; 3(4): 355-360. doi: 10.11648/j.ijnfs.20140304.28. DOI: https://doi.org/10.11648/j.ijnfs.20140304.28
55.Ettebong EO, Nwafor PA, Ekpo M, Ajibesin KK. Contraceptive, estrogenic and anti-estrogenic potentials of methanolic root extract of Carpolobia lutea in rodents. Pak. J. Pharm. Sci. 2011; 24(4): 445-449.
56.Akbaribazm M, Goodarzi N, Rahimi M. Female infertility and herbal medicine: An overview of the new findings. Food Sci Nutr. 2021; 15: 9(10):5869-5882. doi: 10.1002/fsn3.2523. DOI: https://doi.org/10.1002/fsn3.2523
57.Adetunji AO, Price J, Owusu H, Adewale EF, Adesina PA, Saliu TP, Zhu Z, Xedzro C, Asiamah E, Islam S. Mechanisms by which phytogenic extracts enhance livestock reproductive health: current insights and future directions. Front. Vet. Sci. 2025; 12: 1568577. doi: 10.3389/fvets.2025.1568577. DOI: https://doi.org/10.3389/fvets.2025.1568577
58.Rahmawati N, Mohammad AZB, Wahyuni TS, Sani MHM, Kadir AA, Abdullah MNH, Ahmed QU, Zakaria ZA. Medicinal plants from Southeast Asia affecting the reproductive system. Food Bioscience. 2025; 63: 105666. 1qhttps://doi.org/10.1016/j.fbio.2024.105666. DOI: https://doi.org/10.1016/j.fbio.2024.105666
59.Steiner N, Turki RF, El-Khayat W, Malki GA, Tannus S, Dahan MH. Histopathological profile of women who had previously failed in-vitro fertilization and the association to the outcome in the subsequent in-vitro fertilization cycle. Obstet Gynecol. Sci. 2022; 65(1): 64-73. Doi: 10.5468/ogs.21229. DOI: https://doi.org/10.5468/ogs.21229
60.Richardson A, Susie J, Baskind E. Gynaecological pathology and assisted reproductive treatment: can we increase the chances of successful treatment by optimizing the pelvis? Obstet. Gynecol. 2024; 26(3): 139-151. https://doi.org/10.1111/tog.12937. DOI: https://doi.org/10.1111/tog.12937
61.Annapurna P, Pattnaik, N. Evaluation of histopathology of endometrium in primary infertile women, a prospective five years observational study in Konaseema region of Andhra Pradesh. Int’l J Reprod. Contracep. Obstet. Gynecol. 2022; 11(3): 839–843. https://doi.org/10.18203/2320-1770.ijrcog20220566. DOI: https://doi.org/10.18203/2320-1770.ijrcog20220566
62.Borras A, Barral Y, Fabregues F, Casals G, Mora M, Orois A, Mendez M, Saco A, Goday A, Manau D. Histological ovarian features and hormonal determinations in assigned females at birth transgender individuals according to different testosterone preparations. Front. Endocrinol. 2024; 15: 1458846. doi: 10.3389/fendo.2024.1458846. DOI: https://doi.org/10.3389/fendo.2024.1458846
63.Priya K, Manandhar S, Sankhe R, Setty MM, Babu UV, Ranganath-Pai KSR. Structure based virtual docking and molecular dynamics guided identification of potential phytoconstituents from traditionally used female antifertility plant. Pharm Sci. 2021; 28(2): 1-23. doi:10.34172/PS.2021.52. DOI: https://doi.org/10.34172/PS.2021.52
64.Groene N, Nickel A, Rohn AE. Insight on the side effect of female contraceptive products from online drug review: Natural language processing-Based Content Analysis. JMIR AI. 2025; 4: e68809. doi: 10.2196/68809. DOI: https://doi.org/10.2196/68809
65.Stepanenko N, Wolk O, Bianchi E, Wright GJ, Schachter-Safrai N, Makedonski K, Ouro A, Ben-Meir A, Buganim Y, Goldblum A. In silico Docking Analysis for Blocking JUNO-IZUMO1 Interaction Identifies Two Small Molecules that Block in vitro Fertilization. Front Cell Dev Biol. 2022; 5: 10:824629. doi: 10.3389/fcell.2022.824629. DOI: https://doi.org/10.3389/fcell.2022.824629
66.Gunasekaran P, Velmurugan Y, Savaridasson JK, Hemamalini M, Venkatachalam R. In vitro contraceptive activities, molecular docking, molecular dynamics, MM-PBSA, non- covalent interaction and DFT studies of bioactive compounds from Aegle marmelos. Linn., leaves. Front. Chem. 2023; 11: 1096177. DOI: 10.3389/fchem.2023.1096177.
67.Hemamalini M, Venkatachalam R. In vitro contraceptive activities, molecular docking, molecular dynamics, MM-PBSA, non-covalent interaction and DFT studies of bioactive compounds from Aegle marmelos. Linn., leaves. Front Chem. 2023;11: 1096177. doi: 10.3389/fchem. DOI: https://doi.org/10.3389/fchem.2023.1096177