Effects of Saffron (Crocus sativus L.) vs. Folic Acid on Spleen Structure and Function in Intrauterine Growth Restriction
Main Article Content
Abstract
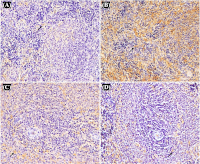
Intrauterine growth restriction (IUGR) is a serious perinatal complication that weakens neonatal immune function, rendering affected infants highly susceptible to infections and consequently increasing their risk of morbidity and mortality. This vulnerability results from impaired development of vital immune organs, particularly the spleen. This study investigated the anti-inflammatory and immunoprotective effects of saffron vs. folic acid supplementation on the spleen of IUGR rat models induced through dietary restriction. Researchers divided 25 rat pups into four groups derived from adult Rattus norvegicus. The third group received saffron (15.68 mg/kg of body weight/day), while the fourth group received folic acid (1.5 mg/kg of body weight/day) from pregnancy through postnatal day 21. The study investigated birth weight, spleen index, histopathology, and tumor necrosis factor-alpha (TNF-α) expression. Birth weight significantly differed between the IUGR control group and those treated with saffron and folic acid. The spleen index showed a significant difference between the IUGR control group and the saffron-treated group. Spleen histopathology analysis revealed significant differences in white pulp components: periarteriolar lymphoid sheath (PALS), follicles, marginal zone, germinal center (GC) and red pulp between the IUGR control group and the group treated with folic acid. There were notable variations in TNF-α expression between the IUGR control group and the groups who received saffron and folic acid. The findings suggest that saffron can potentially increase birth weight and prevent spleen tissue damage due to IUGR. A decrease in inflammatory cytokines in the spleen indicates the organ-protective mechanism, possibly through anti-inflammatory effects.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Kalanjati VP, Wixey JA, Miller SM, Colditz PB, Bjorkman ST. GABAA receptor expression and white matter disruption in intrauterine growth restricted piglets. Int. J. Devl Neuroscience. 2017; 59:1–9. Doi: 10.1016/j.ijdevneu.2017.02.004.
2. Sharma D, Shastri S, Sharma P. Intrauterine Growth Restriction: Antenatal and Postnatal Aspects. Clin Med Insights Pediatr. 2016; 10:67–83. Doi: 10.4137/CMPed.S40070.
3. Alisjahbana B, Rivami DS, Octavia L, Susilawati N, Pangaribuan M, Alisjahbana A, Diana A. Intrauterine growth retardation (IUGR) as determinant and environment as modulator of infant mortality and morbidity: the Tanjungsari Cohort Study in Indonesia. Asia Pac J Clin Nutr. 2019; 28(Suppl 1):S17–31. Doi: 10.6133/apjcn.201901_28(S1).0002.
4. Armengaud JB, Yzydorczyk C, Siddeek B, Peyter AC, Simeoni U. Intrauterine growth restriction: Clinical consequences on health and disease at adulthood. Reprod Toxicol. 2021; 99:168–176. Doi: 10.1016/j.reprotox.2020.10.005.
. Calek E, Binder J, Palmrich P, Eibensteiner F, Thajer A, Kainz T, Harreiter K, Berger A, Binder, C. Effects of Intrauterine Growth Restriction (IUGR) on Growth and Body Composition Compared to Constitutionally Small Infants. Nutrients. 2023; 15(19):4158-4172. Doi: 10.3390/nu15194158.
6. Hu XQ, Zhang L. Hypoxia and Mitochondrial Dysfunction in Pregnancy Complications. Antioxidants (Basel). 2021; 10(3):405-431. Doi: 10.3390/antiox10030405.
7. Ramos-Lopez O, Martinez-Urbistondo D, Vargas-Nuñez JA, Martinez JA. The Role of Nutrition on Meta-inflammation: Insights and Potential Targets in Communicable and Chronic Disease Management. Curr Obes Rep. 2022; 11(4):305–335. Doi: 10.1007/s13679-022-00490-0.
8. Barrea L, Di Somma C, Muscogiuri G, Tarantino G, Tenore GC, Orio F, Colao A, Savastano S. Nutrition, inflammation and liver-spleen axis. Critical Reviews in Food Science and Nutrition. 2018; 58(18):3141–3158. Doi: 10.1080/10408398.2017.1353479.
9. You K, Gu H, Yuan Z, Xu X. Tumor Necrosis Factor Alpha Signaling and Organogenesis. Front Cell Dev Biol. 2021; 9(727075). Doi: 10.3389/fcell.2021.727075.
10. Dapkekar P, Bhalerao A, Kawathalkar A, Vijay N. Risk Factors Associated with Intrauterine Growth Restriction: A Case-Control Study. Cureus. 2023; 15(6):e40178. Doi: 10.7759/cureus.40178.
11. Novitasari EK, Kalanjati VP, Prameswari AS, Hasan NA, Abdurachman A. Effects of Saffron (Crocus sativus L) Compared to Atorvastatin on the Livers of Hypercholesterolemia Rat Models. Trop J Nat Prod Res. 2025; 9(3):1220–1227. Doi: 10.26538/tjnpr/v9i3.43.
12. Pourtau L, Wauquier F, Boutin-Wittrant L, Gaudout D, Moras B, Vignault A, Vaysse C, Richard T, Courtois A, Krisa S, Roux V, Macian N, Pickering G, Wittrant Y. Reduced Production of Pro-Inflammatory and Pro-Catabolic Factors by Human Serum Metabolites Derived from a Patented Saffron Extract Intake. Pharmaceutics. 2024; 16(3):336-353. Doi: 10.3390/pharmaceutics16030336.
13. Sengupta P. The Laboratory Rat: Relating Its Age with Human’s. Int J Prev Med . 2013; 4(6):624-630.
14. Jang EA, Longo LD, Goyal R. Antenatal maternal hypoxia: criterion for fetal growth restriction in rodents. Front Physiol. 2015; 6:176. Doi: 10.3389/fphys.2015.00176.
15. Rejeki PS, Putri EAC, Prasetya RE. Ovariektomi pada Tikus dan Mencit. 1st ed. Airlangga University Press; 2018.
16. Chu X, Ågmo A. Behavioral response is absent under the mating competition in rats (Rattus norvegicus). Physiol Behav. 2019; 201:184–190. Doi: 10.1016/j.physbeh.2019.01.010.
17. Schmidt M, Rauh M, Schmid MC, Huebner H, Ruebner M, Wachtveitl R, Cordasic N, Rascher W, Menendez-Castro C, Hartner A, Fahlbusch FB. Influence of Low Protein Diet-Induced Fetal Growth Restriction on the Neuroplacental Corticosterone Axis in the Rat. Front Endocrinol (Lausanne). 2019; 10:124. Doi: 10.3389/fendo.2019.00124.
18. Sreekantha S, Wang Y, Sakurai R, Liu J, Rehan VK. Maternal food restriction-induced intrauterine growth restriction in a rat model leads to sex-specific adipogenic programming. FASEB J. 2020; 34(12):16073–16085. Doi: 10.1096/fj.202000985RR.
19. Li B, Chang S, Liu C, Zhang M, Zhang L, Liang L, Li R, Wang X, Qin C, Zhang T, Niu B, Wang L. Low Maternal Dietary Folate Alters Retrotranspose by Methylation Regulation in Intrauterine Growth Retardation (IUGR) Fetuses in a Mouse Model. Med Sci Monit. 2019; 25:3354–3365. Doi: 10.12659/MSM.914292.
20. Arunadevi R, Zacharioudaki A, Thorat R, Shenoy SJ, Vijayakumar Sreelatha H. Basic Techniques to Facilitate Small Animal Experimentation. In: Vijayakumar Sreelatha H, Patel S, Nagarajan P, editors. Animal Models in Research: Principles and Practice. Singapore: Springer Nature Singapore; 2024. p. 77–128.
21. Harikrishnan VS. Anaesthesia, Analgesia and Euthanasia of Laboratory Rodents and Rabbits: A Practical Approach. In: Nagarajan P, Gudde R, Srinivasan R, editors. Essentials of Laboratory Animal Science: Principles and Practices. Singapore: Springer; 2021. p. 541–562.
22. Babaei A, Arshami J, Haghparast A, Danesh Mesgaran M. Effects of saffron (Crocus sativus) petal ethanolic extract on hematology, antibody response, and spleen histology in rats. Avicenna J Phytomed. 2014; 4(2):103–109.
23. Zhao M, Chen YH, Dong XT, Zhou J, Chen X, Wang H, Wu S-X, Xia M-Z, Zhang C, Xu D-X. Folic Acid Protects against Lipopolysaccharide-Induced Preterm Delivery and Intrauterine Growth Restriction through Its Anti-Inflammatory Effect in Mice. PLoS One. 2013; 8(12):e82713. Doi: 10.1371/journal.pone.0082713.
24. Liu Y, Huang W, Dai K, Liu N, Wang J, Lu X, Ma J, Zhang M, Xu M, Long X, Liu J, Kou Y. Inflammatory response of gut, spleen, and liver in mice induced by orally administered Porphyromonas gingivalis. J Oral Microbiol. 2022; 14(1):2088936. Doi: 10.1080/20002297.2022.2088936.
25. Tarantino G, Scalera A, Finelli C. Liver-spleen axis: Intersection between immunity, infections and metabolism. World J Gastroenterol. 2013; 19(23):3534–3542. Doi: 10.3748/wjg.v19.i23.3534.
26. Elmore SA. Enhanced Histopathology of the Spleen. Toxicol Pathol. 2006; 34(5):648–655. Doi: 10.1080/01926230600865523.
27. Mashmoul M, Azlan A, Mohtarrudin N, Mohd Yusof BN, Khaza’ai H, Khoo HE, Farzadnia M, Boroushaki MT. Protective effects of saffron extract and crocin supplementation on fatty liver tissue of high-fat diet-induced obese rats. BMC Complement Altern Med. 2016; 16(1):401. Doi: 10.1186/s12906-016-1381-9
28. Cesta MF. Normal Structure, Function, and Histology of the Spleen. Toxicol Pathol. 2006; 34(5):455–465. Doi: 10.1080/01926230600867743.
29. Collins TJ. ImageJ for Microscopy. Biotechniques. 2007; 43(1Suppl):25–30. Doi: 10.2144/000112517.
30. Wang H, Li S, Cui Z, Qin T, Shi H, Ma J, Li L, Yu G. Jiang T, Li C. Analysis of spleen histopathology, splenocyte composition and haematological parameters in four strains of mice infected with Plasmodium berghei K173. Malar J. 2021; 20(1):249. Doi: 10.1186/s12936-021-03786-z.
31. Kyllo HM, Wang D, Lorca RA, Julian CG, Moore LG, Wilkening RB, Rozance PJ, Brown LD, Wesolowski. Adaptive responses in uteroplacental metabolism and fetoplacental nutrient shuttling and sensing during placental insufficiency. Am J Physiol Endocrinol Metab. 2023; 324(6):E556–E568. Doi: 10.1152/ajpendo.00046.2023.
32. Su X, Yuan C, Wang L, Chen R, Li X, Zhang Y, Liu C, Liu X, Liang W, Xing Y. The Beneficial Effects of Saffron Extract on Potential Oxidative Stress in Cardiovascular Diseases. Oxid Med Cell Longev. 2021; 2021:6699821. Doi: 10.1155/2021/6699821.
33. Shi L, Wang Z, Xiao J, Hu R, Zou H, Wang J, Yue Z, Peng Q, Jiang Y, Xue B, Wang L. Folic Acid Alleviates Hydrogen Peroxide-Induced Oxidative Stress in Bovine Placental Trophoblast Cells by Regulating the NRF2/mTOR Signaling Pathway. Int J Mol Sci. 2025; 26(6):2818. Doi: 10.3390/ijms26062818.
34. Lian J, Men Z, Xu X, Li Y, Li J, Wang W, Yao T, Li Y, Qu Y, Feng Y, Wang S. Effects of folic acid supplementation before conception on innate immunity and anti-HBs levels of offspring born to HBsAg-positive mothers. Front Nutr. 2025; 12:1526053. Doi: 10.3389/fnut.2025.1526053.
35. Dai F fang, Hu M, Zhang Y wei, Zhu R hui, Chen L ping, Li Z dian, Huang Y jie, Wei H, Cheng Y xiang. TNF- α /anti-TNF- α drugs and its effect on pregnancy outcomes. Expert Rev Mol Med. 2022; 24:e26. Doi: 10.1017/erm.2022.18.
36. Travis OK, Tardo GA, Giachelli C, Siddiq S, Nguyen HT, Crosby MT, Johnson T, Brown AK, Williams JM, Cornelius DC. Tumor Necrosis Factor-alpha Blockade Improves Uterine Artery Resistance, Maternal Blood Pressure, and Fetal Growth in Placental Ischemic Rats. Pregnancy Hypertens. 2021; 25:39–47. Doi: 10.1016/j.preghy.2021.05.002.
37. Riddle ES, Campbell MS, Lang BY, Bierer R, Wang Y, Bagley HN, Joss-Moore La. Intrauterine Growth Restriction Increases TNFα and Activates the Unfolded Protein Response in Male Rat Pups. J Obes. 2014; 2014:829862. Doi: 10.1155/2014/829862.
38. Ortega MA, Fraile-Martínez O, García-Montero C, Sáez MA, Álvarez-Mon MA, Torres-Carranza D, Alvarez-Mon M, Bujan J, Garcia-Honduvilla N, Bravo C, Guijarro LG, León-Luis JA. The Pivotal Role of the Placenta in Normal and Pathological Pregnancies: A Focus on Preeclampsia, Fetal Growth Restriction, and Maternal Chronic Venous Disease. Cells. 2022; 11(3):568. Doi: 10.3390/cells11030568.
39. Akhtar M, Guo S, Guo Y fang, Zahoor A, Shaukat A, Chen Y, Umar T, Deng G, Guo M. Upregulated-gene expression of pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) via TLRs following NF-κB and MAPKs in bovine mastitis. Acta Trop. 2020; 207:105458. Doi: 10.1016/j.actatropica.2020.105458.
40. Arjmand MH, Hashemzehi M, Soleimani A, Asgharzadeh F, Avan A, Mehraban S, Fakhraei M, Ferns GA, Ryzhikov M, Gharib M, Salari R, Hoseinian SHS, Parizadeh MR, Khazaei M. Therapeutic potential of active components of saffron in post-surgical adhesion band formation. J Tradit Complement Med. 2021; 11(4):328–335. Doi: 10.1016/j.jtcme.2021.01.002.
41. Zhang H, Wang X, Zhang J, Guan Y, Xing Y. Early supplementation of folate and vitamin B12 improves insulin resistance in intrauterine growth retardation rats. Transl Pediatr. 2022; 11(4):466–73. Doi: 10.21037/tp-21-49.
42. Busse M, Plenagl S, Campe NKJ, Müller AJ, Tedford K, Schumacher A, Zenclussen AC. Maternal B Cell-Intrinsic MyD88 Signaling Mediates LPS-Driven Intrauterine Fetal Death. Cells. 2021; 10(10):2693. Doi: 10.3390/cells10102693.
43. Xiong F, Tong Y, You Y, Li P, Huo T, Tu W, Mao M. Prospective cohort study about the lymphocyte subpopulations’ change and impact on the pregnancy outcome in fetal growth restriction. J Matern Fetal Neonatal Med. 2012; 25(12):2773–7. Doi: 10.3109/14767058.2012.715219.
44. Al-Salem AH. Pathophysiology and Functions of the Spleen. In: The Spleen. Springer, Singapore; 2023. p. 33–49. Available from: https://doi.org/10.1007/978-981-99-6191-7_3.
45. Rajković J, Baljak B, Đolai M, Amidžić J. Spleen: Histomorphological Changes of the White Pulp during Fetal Development. J Pharm Pharmacol. 2021; 9:320–332. doi: 10.17265/2328-2150/2021.10.002.
46. White AG, Elias E, Orozco A, Robinson SA, Manners MT. Chronic Stress-Induced Neuroinflammation: Relevance of Rodent Models to Human Disease. Int. J. Mol. Sci. 2024; 25(10):5085. Doi: 10.3390/ijms25105085.
47. Wang Y, Liu J, Burrows PD, Wang JY. B Cell Development and Maturation. In: Wang JY. (eds) B Cells in Immunity and
Tolerance. AEMB, vol 1254. Springer, Singapore. 2020; p.1–22. Available from: https://doi.org/10.1007/978-981-15-3532-1_1
48. White MR, Yates DT. Dousing the flame: reviewing the mechanisms of inflammatory programming during stress-induced intrauterine growth restriction and the potential for ω-3 polyunsaturated fatty acid intervention. Front Physiol. 2023; 14:1250134. Doi: 10.3389/fphys.2023.1250134.
49. Allgäuer L, Cabungcal JH, Yzydorczyk C, Do KQ, Dwir D. Low protein-induced intrauterine growth restriction as a risk factor for schizophrenia phenotype in a rat model: assessing the role of oxidative stress and neuroinflammation interaction. Transl Psychiatry. 2023; 13(1):30. Doi: 10.1038/s41398-023-02322-8.
50. Wong SC, Oh E, Ng CH, Lam KP. Impaired germinal center formation and recall T-cell-dependent immune responses in mice lacking the costimulatory ligand B7-H2. Blood. 2003; 102(4):1381–1388. Doi: 10.1182/blood-2002-08-2416.
51. Weinstein JS, Herman EI, Lainez B, Licona-Limón P, Esplugues E, Flavell R, Craft J. TFH cells progressively differentiate to regulate the germinal center response. Nat Immunol. 2016; 17(10):1197–1205. Doi: 10.1038/ni.3554.
52. Klei TRL, Meinderts SM, van den Berg TK, van Bruggen R. From the Cradle to the Grave: The Role of Macrophages in Erythropoiesis and Erythrophagocytosis. Front Immunol. 2017; 8:73. Doi: 10.3389/fimmu.2017.00073.
53. Nagelkerke SQ, Bruggeman CW, den Haan JMM, Mul EPJ, van den Berg TK, van Bruggen R, Kuijpers TW. Red pulp macrophages in the human spleen are a distinct cell population with a unique expression of Fc-γ receptors. Blood Adv. 2018; 2(8):941–953. Doi: 10.1182/bloodadvances.2017015008.
54. Kirici P, Çağıran FT, Kali Z, Tanriverdi ES, Mavral N, Ecin SM. Determination of maternal serum pro-inflammatory cytokine changes in intrauterine growth restriction. Eur Rev Med Pharmacol Sci. 2023; 27:1996-2001.