Naphthoquinones Isolated from Fusarium solani, an Endophytic Fungi of Cola nitida, with Potentials for Pharmaceutical and Industrial Applications
Main Article Content
Abstract
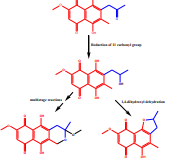
Endophytic fungi isolated from Nigeria Rainforest medicinal plants have been shown to possess potentials as sources of novel therapeutic agents. In our effort to further explore the rainforest medicinal plants in Nigeria for endophytic fungi populations, we investigated Cola nitida for the isolation of novel fungal endophytes that can synthesize unique compounds of pharmaceutical and industrial importance. The endophytic fungus was isolated from the leaves of Cola nitida using standard protocols. The identification was carried out by DNA amplification and sequencing of the internal transcribed spacer (ITS) region. The pure fungus was grown using solid fermentation on a rice medium and the metabolites were extracted using ethyl acetate. The crude extract was subjected to several chromatographic techniques to isolate compounds 1-4. The structures of these compounds were elucidated using a combination of 1-and 2-D NMR and Mass Spectrometry. The fungus was identified as Fusarium solani and the isolated compounds were elucidated as Javanicin (1), Solaniol (2), 3-O-methyl fusarubin (3) and anhydrojavanicin (4). This study further contributes to the plethora of endophytic fungi isolated from the leaves of Cola nitida. The isolated naphthoquinones have potential for development into novel therapeutic agents as well as applications as pigments in food, cosmetics, pharmaceutical, and textile industries.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Nwobodo DC, Okoye NN, Sifir Mudkhur M, Ikem JC, Eze PM, Okoye FBC, Saki M, Esimone CO. In vitro antiplasmodial and anticancer analyses of endophytic fungal extracts isolated from selected Nigerian medicinal plants. Sci. Rep. 2024; 14(01): 19765. doi: 10.1038/s41598-024-66456-5. DOI: https://doi.org/10.1038/s41598-024-66456-5
2. Tiwari P, Bae H. Endophytic Fungi: Key Insights, Emerging Prospects, and Challenges in Natural Product Drug Discovery. Microorganisms. 2022; 10(02): 360. https://doi.org/10.3390/microorganisms10020360 DOI: https://doi.org/10.3390/microorganisms10020360
3. Wang H, Eze PM, Hӧfert S, Janiak C, Hartmann R, Okoye FBC, Esimone CO, Orfali RS, Dai H, Liu Z, Proksch P. Substituted L-tryptophan-L-phenyllactic acid conjugates produced by an endophytic fungus Aspergillus aculeatus using an OSMAC approach. RSC Adv. 2018; 8(14):7863–7872. DOI: https://doi.org/10.1039/C8RA00200B
4. Okoye FBC, Lu S, Nworu CS, Esimone CO, Proksch P, Chaldi A. Debbab A. Depsidone and Diaryl Ether Derivatives from the Fungus Corynespora cassiicola, an Endophyte of Gongronema latifolium. Tetrahedron Lett. 2013; 54(32): 4210–4214. DOI: https://doi.org/10.1016/j.tetlet.2013.05.117
5. Okoye FBC, Nworu CS, Debbab A, Esimone CO, Proksch P. Two new Cytochalasins from an endophytic fungus, KL-1.1 isolated from Psidium guajava leaves. Phytochem. Lett. 2015; 14: 51-55. DOI: https://doi.org/10.1016/j.phytol.2015.09.004
6. Fasinu PS, Okoye FBC, Abiodun OO, Kamdem RST, Ogbole OO. Editorial: Fungal Bioactive Metabolites of Pharmacological Relevance. Front. Pharmacol. 2022; 13:912068. doi: 10.3389/fphar.2022.912068 DOI: https://doi.org/10.3389/fphar.2022.912068
7. Obidiegwu CO, Abba CC, Okoye NN, Okolo CC, Nwachukwu CU, Ujam NT, Okoye FBC. ACL-4, an Endophytic Fungus Isolated from Ageratum conyzoides Leaves Possesses the Unique Potential of Generating Low Molecular Weight Bioactive Lead Compounds. Trop. J. Nat. Prod. Res. 2022; 6(12): 2041-2046
8. Gupta S, Chaturvedi P, Kulkarni MG, Van Staden J. A critical review on exploiting the pharmaceutical potential of plant endophytic fungi. Biotech. Adv. 2020; 39(01):107462. doi: 10.1016/j.biotechadv.2019.107462 DOI: https://doi.org/10.1016/j.biotechadv.2019.107462
9. Liao C, Doilom M, Jeewon R, Hyde KD, Manawasinghe IS, Thilini Chethana KW, Balasuriya A, Thakshila SAD, Luo M, Mapook A, Htet ZH, Koodalugodaarachchi V, Wijekoon N, Saxena RK, Senanayake IC, Kularathnage ND, Alrefaei AF, Dong W. Challenges and update on fungal endophytes: classification, definition, diversity, ecology, evolution and functions. Fung. Divers. 2025; 131(08): 301–367. https://doi.org/10.1007/s13225-025-00550-5 DOI: https://doi.org/10.1007/s13225-025-00550-5
10. Maharjan S, Lee SB, Kim GJ, Cho SJ, Nam JW, Chin J, Choi H. Isolation of Unstable Isomers of Lucilactaene and Evaluation of Anti-Inflammatory Activity of Secondary Metabolites Produced by the Endophytic Fungus Fusarium sp. QF001 from the Roots of Scutellaria baicalensis. Molecules 2020; 25(04):923. doi: 10.3390/molecules25040923 DOI: https://doi.org/10.3390/molecules25040923
11. Adione NM, Onyeka IP, Abba CC, Okoye NN, Eze PM, Umeokoli BO, Anyanwu OO, Okoye FBC. Antimicrobial Activities of the Endophytic Fungus, Fusarium equiseti, Isolated from the leaves of Ocimum gratissimum. J. Adv. Med. Pharm. Sci. 2022; 24(07): 11-23 DOI: https://doi.org/10.9734/jamps/2022/v24i730312
12. Ebrahim W, Özkaya FC, Ebada SS. Antifungal metabolites from endophytic fungus Fusarium verticillioides strain WF18. South Afr. J Bot. 2020; 133(01): 40–44. DOI: https://doi.org/10.1016/j.sajb.2020.06.029
13. Ngwoke KG, Tochukwu N, Ekwealor C, Nwankwo V, Obi-Okafor U, Izundu C, Okoye FBC, Esimone C, Proksch P, Situ C. Antibacterial, anti-inflammatory and peroxidase-mediated cyclooxygenase-1 inhibitory properties of Fusarium solani extract. Pharm. Bio. 2019; 57(01): 310-317. DOI: https://doi.org/10.1080/13880209.2019.1606260
14. Khan N, Afroz F, Begum MN, Roy Rony S, Sharmin S, Moni F, Mahmood Hasan C, Shaha K, Sohrab MH. Endophytic Fusarium solani: A rich source of cytotoxic and antimicrobial napthaquinone and aza-anthraquinone derivatives. Toxicol. Rep. 2018; 5: 970–976. DOI: https://doi.org/10.1016/j.toxrep.2018.08.016
15. Ravichandiran P, Sheet S, Premnath D, Kim AR, Yoo DJ. 1,4-Naphthoquinone Analogues: Potent Antibacterial Agents and Mode of Action Evaluation. Molecules 2019; 24(07): 1437. DOI: https://doi.org/10.3390/molecules24071437
16. Liu H, Yan C, Li C, You T, She Z. Naphthoquinone Derivatives with Anti-Inflammatory Activity from Mangrove-Derived Endophytic Fungus Talaromyces sp. SK-S009. Molecules 2020; 25(03): 576. DOI: https://doi.org/10.3390/molecules25030576
17. Adesanwo JK, Ogundele SB, Akinpelu DA, McDonald AG. Chemical Analyses, Antimicrobial and Antioxidant Activities of Extracts from Cola nitida Seed. J. Explor. Res. Pharmacol. 2017; 2(03): 67–77. DOI: https://doi.org/10.14218/JERP.2017.00015
18. Ekalu A, Habila JD. Phytochemistry, pharmacology and medicinal uses of Cola (Malvaceae) family: a review. Med. Chem. Res. 2020; 29(12): 2089–2105. DOI: https://doi.org/10.1007/s00044-020-02637-x
19. Nwobodo DC, Ihekwereme CP, Okoye FBC. Screening of endophytic fungal metabolites from Cola nitida leaves for antimicrobial activities against clinical isolates of Pseudomonas aeruginosa. The EuroBiotech J. 2020; 4(03): 161–166. DOI: https://doi.org/10.2478/ebtj-2020-0019
20. Damour H, Okoye FBC, Proksch P, Hakiki A, Mosaddak M, Hegazy MF, Debbab A. Pretrichodermamide A and nafuredin from Trichoderma sp, an endophyte of Cola nitida. J. Mat. and Environ. Sci. 2015; 6(03): 779–783.
21. Nwobodo DC, Ugwu MC, Chuka M, Okoye FBC, Esimone CO. Antimicrobial and Antioxidant Compounds Produced by Fungal Endophytes Isolated from Selected Nigerian Medicinal Plants. Int. J. Pharm. Phytopharmacol. Res. 2023; 13(03): 6-15 DOI: https://doi.org/10.51847/7hNZ3qg4m4
22. Tatum JH, Baker RA, Berry RE. Metabolites of Fusarium solani. Phytochem. 1989; 28(01): 283-284 DOI: https://doi.org/10.1016/0031-9422(89)85062-9
23. Wu, W., Wang, S., Zhang, H, Guo W, Lu H, Xu H, Zhan R, Fidan O, Sun L. Biosynthesis of Novel Naphthoquinone Derivatives in the Commonly-used Chassis Cells Saccharomyces cerevisiae and Escherichia coli. Appl. Biochem. Microbiol. 2021; 57 (S1), S11–S26. https://doi.org/10.1134/S0003683821100124 DOI: https://doi.org/10.1134/S0003683821100124
24. Kristensen SB, Pedersen TB, Nielsen MR, Wimmer R, Muff J, Sørensen JL. Production and Selectivity of Key Fusarubins from Fusarium solani due to Media Composition. Toxins 2021; 13(06): 376; https://doi.org/10.3390/toxins13060376 DOI: https://doi.org/10.3390/toxins13060376
25. Shah A, Rather MA, Hassan QP, Aga MA, Mushtaq S, Shah AM, Hussain A, Baba SA, Ahmad Z. Discovery of anti-microbial and anti-tubercular molecules from Fusarium solani : an endophyte of Glycyrrhiza glabra. J. Appl. Microbiol. 2017; 122(05): 1168–1176. DOI: https://doi.org/10.1111/jam.13410
26. Yang Z, Ding J, Ding K, Chen D, Cen S, Ge M. (2013). Phomonaphthalenone A: A novel dihydronaphthalenone with anti-HIV activity from Phomopsis sp. HCCB04730. Phytochem. Lett. 2013; 6(02):257–260. DOI: https://doi.org/10.1016/j.phytol.2013.02.003
27. Arsenault GP. Fungal metabolites—III. Quinones from Fuasrium solani D2 Purple and Structure of (+)-Solaniol. Tetrahedron 1968; 24(13): 4745–4749. DOI: https://doi.org/10.1016/S0040-4020(01)98671-5
28. Choi HG, Song JH, Park M, Kim S, Kim CE, Kang KS, Shim SH. Neuroprotective γ-Pyrones from Fusarium Solani JS-0169: Cell-Based Identification of Active Compounds and an Informatics Approach to Predict the Mechanism of Action. Biomolecules 2020, 10(01): 1–11. DOI: https://doi.org/10.3390/biom10010091
29. Meyer GW, Bahamon Naranjo MA, Widhalm JR. Convergent evolution of plant specialized 1,4-naphthoquinones: metabolism, trafficking, and resistance to their allelopathic effects. J. Experim. Bot., 2021; 72(02):167–176. DOI: https://doi.org/10.1093/jxb/eraa462
30. Naysmith BJ, Hume PA, Sperry J, Brimble MA. Pyranonaphthoquinones – isolation, biology and synthesis: an update. Nat. Prod. Rep. 2017; 34(01): 25–61. DOI: https://doi.org/10.1039/C6NP00080K
31. Kumar KP, Javvaji K, Poornachandra Y, Allanki AD, Misra S. Antimicrobial, Anti-plasmodial and Cytotoxicity Properties of Bioactive Compounds from Fusarium sp. USNPF102. J. Microbiol. Res. 2017; 7(02): 23–30.
32. Kyekyeku JO, Kusari S, Adosraku RK, Bullach A, Golz C, Strohmann C, Spiteller M. Antibacterial secondary metabolites from an endophytic fungus, Fusarium solani JK10. Fitoterapia 2017; 119: 108–114. https://doi.org/10.1016/j.fitote.2017.04.007 DOI: https://doi.org/10.1016/j.fitote.2017.04.007
33. Jiang CX, Li J, Zhang JM, Jin XJ, Yu B, Fang JG, Wu QX. Isolation, Identification, and Activity Evaluation of Chemical Constituents from Soil Fungus Fusarium avenaceum SF-1502 and Endophytic Fungus Fusarium proliferatum AF-04. J. Agric. Food Chem. 2019; 67(07): 1839–1846. DOI: https://doi.org/10.1021/acs.jafc.8b05576
34. Bezabih M, Abegaz BM, Dufall K, Croft K, Skinner-Adams T, Davis TME. Antiplasmodial and Antioxidant Isofuranonaphthoquinones from the Roots of Bulbine capitata. Planta Med. 2001; 67(04): 340–344. DOI: https://doi.org/10.1055/s-2001-14329
35. Lebeau J, Petit T, Clerc P, Dufossé L, Caro Y. Isolation of two novel purple naphthoquinone pigments concomitant with the bioactive red bikaverin and derivates thereof produced by Fusarium oxysporum. Biotech. Progr. 2019; 35(01): 1–13. DOI: https://doi.org/10.1002/btpr.2738
36. Menezes BS, Solidade LS, Conceição AA, Santos Junior MN, Leal PL, de Brito ES, Canuto KM, Mendonça S, de Siqueira FG, Marques LM. Pigment production by Fusarium solani BRM054066 and determination of antioxidant and anti-inflammatory properties. AMB Express 2020; 10(01): 117. doi: 10.1186/s13568-020-01054-y. DOI: https://doi.org/10.1186/s13568-020-01054-y
37. Di Salvo E, Lo Vecchio G, De Pasquale R, De Maria L, Tardugno R, Vadalà R, Cicero N. Natural Pigments Production and Their Application in Food, Health and Other Industries. Nutrients. 2023; 15(08):1923. doi: 10.3390/nu15081923. DOI: https://doi.org/10.3390/nu15081923
38. Valenzuela-Gloria MS, Balagurusamy N, Chávez-González ML, Aguilar O, Hernández-Almanza A, Aguilar CN. Molecular Characterization of Fungal Pigments. J. Fungi 2021; 7(05): 326. DOI: https://doi.org/10.3390/jof7050326
39. Guo R, Cai X, Li Q, Huang Y, Chen B, Guan P, Tang J, Zou X. An efficient high‐speed countercurrent chromatography method for preparative separation of javanicin from Fusarium solani, a fungus isolated from the fruiting body of the mushroom Trametes trogii. Biomed. Chromat. 2019; 33(09):e4574. doi: 10.1002/bmc.4574. DOI: https://doi.org/10.1002/bmc.4574
40. Mone NS, Bhagwat SA, Sharma D, Chaskar M, Patil RH, Zamboni P, Nawani NN, Satpute SK. Naphthoquinones and Their Derivatives: Emerging Trends in Combating Microbial Pathogens. Coatings. 2021; 11(04):434. https://doi.org/10.3390/coatings11040434 DOI: https://doi.org/10.3390/coatings11040434