Piliostigma thonningii (Schum.) Milne-Redh: GC-MS profiling, In vitro, In vivo Antioxidant Potential and Toxicological Assessment in Female Wistar Rats
Main Article Content
Abstract
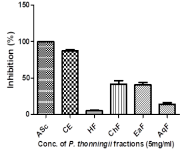
Medicinal plants showed promising prospects in managing various diseases due to their rich bioactive content with Piliostigma thonningii being a notable example. This study evaluated the plant’s in vitro, in vivo antioxidant potential, GC-MS profiling of its crude extract as well as the toxicological assessment to ascertain the plants toxicological profile in female wistar rat for a putative lead against uterine fibroids. GC-MS analysis identified 31 compounds, with 1,3-Dioxane-2-pentadecyl- (47.03%) as the most abundant. In vitro assays revealed that the crude extract (CE), chloroform (ChF), & ethyl-acetate fractions (EaF) demonstrated high Ferric reducing antioxidant power (FRAP) & 1,1- diphenyl-2-picrylhydrazyl (DPPH) activities, while n-hexane (HF) & aqueous fractions (AqF) presented greater Cupric ion Reducing Antioxidant Capacity (CUPRAC) activity. CE and EaF demonstrated low IC50 values for FRAP, while CE and ChF gave the lowest IC50 values for DPPH and finally, HF, showed better CUPRAC compared to the standard. Acute toxicity assessment of the plant’s crude extract was evaluated at varying doses of 500, 1000 and 2000 mg/kg body weight and the results showed no significant changes in endogenous antioxidant enzymes, suggesting the plant does not induce oxidative stress in rats. The extract was not toxic to both kidney and liver as reported in previous studies. Histological analysis revealed normal ovarian architecture at 500 and 1000 mg/kg but tissue distortion at 2000 mg/kg. P. thonningii leaves exhibit high antioxidant activity, aligning with previous reports, and showed no oxidative stress to female wistar rats except at high dose (2000mg/kg b.w). Its high antioxidant potential maybe useful in managing oxidative stress associated with uterine fibroids.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Škrovánková S, Mišurcová L, Machů L. Antioxidant activity and protecting health effects of common medicinal plants. Adv Food Nutr Res. 2012; 67:75-139. https://doi.org/10.1016/B978-0-12-394598-3.00003-4. DOI: https://doi.org/10.1016/B978-0-12-394598-3.00003-4
2. WHO, IUCN, &WWF. Guidelines on the conservation of medicinal plants. Gland, Switzerland, 1993; 38.
3. Oladele JO, Ajayi EI, Oyeleke OM, Oladele OT, Olowookere BD, Adeniyi BM, Oyewole OI, Oladiji AT. A systematic review on COVID-19 pandemic with special emphasis on curative potentials of Nigeria based medicinal plants. Heliyon. 2020; 1:6(9). DOI: https://doi.org/10.1016/j.heliyon.2020.e04897
4. AlAshqar A, Lulseged B, Mason-Otey A, Liang J, Begum UA, Afrin S, Borahay MA. Oxidative stress and antioxidants in uterine fibroids: pathophysiology and clinical implications. Antiox. 2023; 12(4):807. https://doi.org/10.3390/antiox12040807 DOI: https://doi.org/10.3390/antiox12040807
5. Soibi-Harry AP, Makwe CC, Oluwole AA, Garba SR, Ajayi AT, Anyanwu R, Anorlu RI. Serum Oxidative Stress Markers in Women with Uterine Fibroids in Lagos, Nigeria. medRxiv. 2021:2021-07.https://doi.org/10.1101/2021.07.07.21260056 DOI: https://doi.org/10.1101/2021.07.07.21260056
6. Manessis G, Kalogianni AI, Lazou T, Moschovas M, Bossis I, Gelasakis AI. Plant-derived natural antioxidants in meat and meat products. Antiox. 2020; 9(12):1215. https://doi.org/10.3390/antiox9121215 DOI: https://doi.org/10.3390/antiox9121215
7. Kıran TR, Otlu O, Karabulut AB. Oxidative stress and antioxidants in health and disease. J. Lab. Med. 2023; 47(1):1-1. https://doi.org/10.1515/labmed-2022-0108 DOI: https://doi.org/10.1515/labmed-2022-0108
8. Nwozo OS, Effiong EM, Aja PM, Awuchi CG. Antioxidant, phytochemical, and therapeutic properties of medicinal plants: A review. Int J. Food Prop. 2023; 26(1):359-388.. https://doi.org/10.1080/10942912.2022.2157425 DOI: https://doi.org/10.1080/10942912.2022.2157425
9. Popoola OO. Phenolic compounds composition and in vitro antioxidant activity of Nigerian Amaranthus viridis seed as affected by autoclaving and germination. Meas Food. 2022;6:100028.. https://doi.org/10.1016/j.meafoo.2022.100028 DOI: https://doi.org/10.1016/j.meafoo.2022.100028
10. Adegbola PI, Adetutu A, Olaniyi TD. Antioxidant activity of Amaranthus species from the Amaranthaceae family – A review. S Afr J. Bot. 2020;133:111-117.. https://doi.org/10.1016/j.sajb.2020.07.003 DOI: https://doi.org/10.1016/j.sajb.2020.07.003
11. Ngozi P Okolie, Falodun A and Oluseyi Davids. Evaluation of the antioxidant activity of root extract of pepper fruit (Dennetia tripetala), and its potential for the inhibition of Lipid peroxidation. Afri J Trad Complem and Altern Med 2014; 11(3):221-227. 10.4314/ajtcam.v11i3.31 DOI: https://doi.org/10.4314/ajtcam.v11i3.31
12. Odion EE, Falodun A and Adelusi SA. Total flavonoid, Total Phenolic and antioxidant potential of root bark extract and fractions of from Cola rostrata (Sterculiaceae) K. Schum. University of Benin 2013; J. Sci. Tech 1 (2): 38 - 42.
13. Marquardt P, Vissiennon C, Schubert A, Birkemeyer C, Ahyi V, Fester K. In vitro anti-inflammatory and antimicrobial activity of Piliostigma thonningii leaf extracts from Benin. Planta Med. 2020; 86(17):1269-1277. https://doi.org/10.1055/a-1229-4565 DOI: https://doi.org/10.1055/a-1229-4565
14. Ighodaro OM, Omole JO. Effects of Nigerian Piliostigma thonningii species leaf extract on lipid profile in Wistar rats. Int Sch Res Notices. 2012; 2012:387942. https://doi.org/10.5402/2012/387942 DOI: https://doi.org/10.5402/2012/387942
15. Dagallier LP, Janssens SB, Dauby G, Blach‐Overgaard A, Mackinder BA, Droissart V, Svenning JC, Sosef MS, Stévart T, Harris DJ, Sonké B. Cradles and museums of generic plant diversity across tropical Africa. New Phytol. 2020; 5(5):2196-213. https://doi.org/10.1111/nph.16293 DOI: https://doi.org/10.1111/nph.16293
16. Son NT, Le TA, Thuy DT, Nguyen DL, Tuyen TT, Hoang Thi MD, Nguyen MH. Essential oils of the Leaf and Stem of Polyalthia Viridis Craib and their biological activities. Nat. Prodt. Commun. 2021;16(9):1934578X211032011. DOI: https://doi.org/10.1177/1934578X211032011
17. Ibewuike JC, Ogundaini AO, Ogungbamila FO, Martin MT, Gallard JF, Bohlin L, Païs M. Piliostigmin, a 2-phenoxychromone, and C-methylflavonols from Piliostigma thonningii. Phytochem. 1996; 43(3):687-690. https://doi.org/10.1016/0031-9422(96)00367-6. DOI: https://doi.org/10.1016/0031-9422(96)00367-6
18. Aworinde D, Erinoso S, Ibukunoluwa M, Teniola S. Herbal concoctions used in the management of some women-related health disorders in Ibadan, Southwestern Nigeria. J Appl Biosci. 2020; 147(1):15091-15099. https://doi.org/10.35759/JABs.147.2. DOI: https://doi.org/10.35759/JABs.147.2
19. Olayemi DO, Onakpa MM, Jegede OC. Egg Hatch Assay and Lavicidal Activity of Piliostigma Thonningii Pod Extract and Fractions on Haemonchus contortus. Tanz. Vet. J. 2020;35(1).
20. Akinpelu DA, Obuotor EM. Antibacterial activity of Piliostigma thonningii stem bark. Fitoterapia. 2000; 71(4):442-443. https://doi.org/10.1016/S0367-326X(00)00136-2. DOI: https://doi.org/10.1016/S0367-326X(00)00136-2
21. Afolayan M, Srivedavyasasri R, Asekun OT, Familoni OB, Orishadipe A, Zulfiqar F, Ross SA. Phytochemical study of Piliostigma thonningii, a medicinal plant grown in Nigeria. Med Chem Res. 2018; 27:2325-2330. https://doi.org/10.1007/s00044-018-2238-1. DOI: https://doi.org/10.1007/s00044-018-2238-1
22. Dasofunjo K, Nwodo FO, Ipav SS, Barminas ZL. Effect of the ethanolic extract of Piliostigma thonningii leaves on kidney function indices and haematological parameters of male albino Wistar rats. J. Nat. Prod. Plant Resour., 2012; 2 (6):670-674
23. Agab SA, EL-Kamali HH, Ibrahim MA, Nour AA, Al Foraih M, Kheiralla KE, Shanwaz MA, Elsamani MO, Ali OY, Mashnafi SQ, Omer EA. The Impact of Aqueous Piliostigma thonningii Fruit Extract on Certain Biochemical Parameters on Male Wister Albino Rats. Egypt Acad J Biol Sci C Physiol Mol Biol 2024; 16(2):229-36. 10.21608/eajbsc.2024.387169 DOI: https://doi.org/10.21608/eajbsc.2024.387169
24. Nurudeen QO, Yusuf ZM, Salimon SS, Falana MB, Asinmi MR, Elemosho AO, Dikwa MA. Toxicological status of the hydroethanolic extract of Piliostigma thonningii leaves in female Wistar rats. Niger J. Biotechnol. 2023; 40(1):92-99. https://doi.org/10.4314/njb.v40i1.11. DOI: https://doi.org/10.4314/njb.v40i1.11
25. Ibe CI, Ajaegbu EE, Ajaghaku AA, Eze PM, Onyeka IP, Ezugwu CO, Okoye FBC. In vitro and in vivo antioxidant potential of the methanol extract, its fractions and isolated compounds of Piliostigma thonningii. Phytomed Plus. 2022; 2(4):100335. https://doi.org/10.1016/j.phyplu.2022.100335. DOI: https://doi.org/10.1016/j.phyplu.2022.100335
26. Dasofunjo K, Nwodo OFC, Yakubu OE, Ejoba R, Ukpanukpong RU, Ipav SS, Girgi SL. Hepatoprotective effect of Piliostigma thonningii leaves on male Wistar albino rats. Asian J Plant Sci Res. 2013; 3(4):13-17.
27. Ajiboye TO, Salawu NA, Yakubu MT, Oladiji AT, Akanji MA, Okogun JI. Antioxidant and drug detoxification potentials of Hibiscus sabdariffa anthocyanin extract. Drug Chem Toxicol. 2011; 34(2):109-115. https://doi.org/10.3109/01480545.2010.536767. DOI: https://doi.org/10.3109/01480545.2010.536767
28. Martin MT, Pais M, Ogundaini AO, Ibewuike JC, Ogungbamila FO. Complete 1H and 13C NMR assignment of a kaurane diterpene from Piliostigma thonningii. Magn Reson Chem. 1997; 35(12):896-898. https://doi.org/10.1002/(SICI)1097-458X(199712)35:12<896::AID-OMR150>3.0.CO;2-S. DOI: https://doi.org/10.1002/(SICI)1097-458X(199712)35:12<896::AID-OMR150>3.0.CO;2-S
29. Baratta MT, Ruberto G, Tringali C. Constituents of the pods of Piliostigma thonningii. Fitoterapia. 1999; 70(2):205-208. https://doi.org/10.1016/S0367-326X(98)00035-5 DOI: https://doi.org/10.1016/S0367-326X(98)00035-5
30. Garba AN. Preliminary Phytochemical Screening and Antimicrobial Activity of Piliostigma thonningii Ethanol Stem Bark Extract 2024. Available at SSRN: https://ssrn.com/abstract=4751226 or http://dx.doi.org/10.2139/ssrn.4751226 DOI: https://doi.org/10.2139/ssrn.4751226
31. Sumaiyah S, Masfria M, Rusdi I, Dalimunthe A. The preparation and characterization of ethanol extract nanoparticle from Rhaphidophora pinnata (Lf) Schott leaves by using Arabic gum and dextrin. J. Young Pharm. 2018; 10(2s):S84-S86. https://doi.org/10.5530/jyp.2018.2s.16 DOI: https://doi.org/10.5530/jyp.2018.2s.16
32. Hostettmann K, Hostettmann M, Marston A. Saponins, in terpenoids. In: Charlwood BV, Banthorpe DV, editors. Methods in Plant Biochemistry. Vol. 7. San Diego, CA: Academic Press; 1991. 435-471.
33. Anyanwu GO, Iqbal J, Khan SU, Zaib S, Rauf K, Onyeneke CE, Ojo OO. Antidiabetic activities of chloroform fraction of Anthocleista vogelii Planch root bark in rats with diet- and alloxan-induced obesity-diabetes. J. Ethnopharmacol. 2019; 229:293-302. https://doi.org/10.1016/j.jep.2018.10.021. DOI: https://doi.org/10.1016/j.jep.2018.10.021
34. Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of "antioxidant power": the FRAP assay. Anal Biochem. 1996; 239(1):70-76. https://doi.org/10.1006/abio.1996.0292. DOI: https://doi.org/10.1006/abio.1996.0292
35. Xu BJ, Chang SK. A comparative study on phenolic profiles and antioxidant activities of legumes as affected by extraction solvents. J Food Sci. 2007;72(2):S159-S166. https://doi.org/10.1111/j.1750-3841.2006.00260.x DOI: https://doi.org/10.1111/j.1750-3841.2006.00260.x
36. Apak R, Güçlü K, Demirata B, Özyürek M, Çelik SE, Bektaşoğlu B, Özyurt D. Comparative evaluation of various total antioxidant capacity assays applied to phenolic compounds with the CUPRAC assay. Mol. 2007;12(7):1496-1547. https://doi.org/10.3390/12071496. DOI: https://doi.org/10.3390/12071496
37. Hadwan MH. New method for assessment of serum catalase activity. Indian J Sci Technol. 2016;9(4):1-5. https://doi.org/10.17485/ijst/2016/v9i4/80499 DOI: https://doi.org/10.17485/ijst/2016/v9i4/80499
38. Li X. Improved pyrogallol autoxidation method: a reliable and cheap superoxide-scavenging assay suitable for all antioxidants. J Agric Food Chem. 2012;60(25):6418-6424. https://doi.org/10.1021/jf204970r DOI: https://doi.org/10.1021/jf204970r
39. Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70-77. DOI: https://doi.org/10.1016/0003-9861(59)90090-6
40. Gérard-Monnier D, Erdelmeier I, Régnard K, Moze-Henry N, Yadan JC, Chaudiere J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem Res Toxicol. 1998;11(10):1176-1183. https://doi.org/10.1021/tx9701790. DOI: https://doi.org/10.1021/tx9701790
41. Gamde SM, Kabiru H, Abdulazziz A, Abubakar KA, Musa AA, Perede A. Histopathological and biochemical effects of aqueous leaf extract of Cadaba farinosa on the liver of adult Wistar rats. Int J Res Med Sci. 2019;7(12):4329-4334. https://doi.org/10.18203/2320-6012.ijrms20194298. DOI: https://doi.org/10.18203/2320-6012.ijrms20194298
42. Yinusa I. Phytochemical screening, GC-MS and FTIR analysis of ethanol extract of Piliostigma thonningii (Schum. Milne Redth) leaf. Commun Phys Sci. 2010;5(1, 2, 3).
43. Radhamani T, Britto JS. GC-MS analysis of Polygala arillata Buch–Ham ExD. Ann Biol Res. 2013;4(11):70-75.
44. Sermakkani M, Thangapandian V. GC-MS analysis of Cassia italica leaf methanol extract. Asian J Pharm Clin Res. 2012;5(2):90-94. https://api.semanticscholar.org/CorpusID:32646987.
45. Aparna V, Dileep KV, Mandal PK, Karthe P, Sadasivan C, Haridas M. Anti‐inflammatory property of n‐hexadecanoic acid: structural assessment. Chem Biol Drug Des. 2012;80(3):434-439. https://doi.org/10.1111/j.1747-0285.2012.01418.x. DOI: https://doi.org/10.1111/j.1747-0285.2012.01418.x
46. Bruce SO, Onyemailu VO, Orji CE. Evaluation of the antiulcer activity and GC-MS spectroscopic analysis of the crude ethanolic extract of Pueraria phaseoloides leaf (Roxb) Benth. (FABACEAE). World J Pharm Res. 2021;10(7):39-59. https://doi.org/10.20959/wjpr20217-20585
47. Prajna PS, Rama BP, Sunil K. Identification of bioactive compound in Loeseneriella arnottiana Wright root by GC-MS analysis. World J Pharm Res. 2016;5(4):1559-1569. https://doi.org/10.20959/wjpr20164-6013
48. Komansilan A, Abadi AL, Yanuwiadi B, Kaligis DA. Isolation and identification of biolarvicide from soursop (Annona muricata Linn) seeds to mosquito (Aedes aegypti) larvae. Int J Eng Technol. 2012;12(03):28-32. https://api.semanticscholar.org/CorpusID:296141.
49. Dalla Lana DF, Batista BG, da Rosa Machado G, Teixeira ML, de Oliveira LFS, Machado MM, et al. Design, synthesis, and evaluation of novel 2-substituted 1,4-benzenediol library as antimicrobial agents against clinically relevant pathogens. Saudi Pharm J. 2019;27(8):1064-1074. https://doi.org/10.1016/j.jsps.2019.09.003. DOI: https://doi.org/10.1016/j.jsps.2019.09.003
50. Deryabin DG, Tolmacheva AA. Antibacterial and anti-quorum sensing molecular composition derived from Quercus cortex (Oak bark) extract. Mol. 2015;20(9):17093-17108. https://doi.org/10.3390/molecules200917093. DOI: https://doi.org/10.3390/molecules200917093
51. Sales-Campos H, Reis de Souza P, Crema Peghini B, Santana da Silva J, Ribeiro Cardoso C. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev Med Chem. 2013;13(2):201-210. https://doi.org/10.2174/138955713804805193. DOI: https://doi.org/10.2174/1389557511313020003
52. Rao BG, Rao ES, Rao TM. Quantification of phytochemical constituents and in-vitro antioxidant activity of Mesua ferrea leaves. Asian Pac J Biomed. 2012;2(2):539-542. https://doi.org/10.1016/S2221-1691(12)60269-X. DOI: https://doi.org/10.1016/S2221-1691(12)60269-X
53. Imo C, Yakubu OE, Imo NG, Udegbunam IS, Onukwugha OJ. Chemical composition of Xylopia aethiopica fruits. Am J Physiol Biochem Pharmacol. 2018;7(2):48-53. doi:10.5455/ajpbp.20180521064020 DOI: https://doi.org/10.5455/ajpbp.20180521064020
54. Abdallah HM, El-Bassossy HM, El-Halawany AM, Ahmed TA, Mohamed GA, Malebari AM, Hassan NA. Self-nanoemulsifying drug delivery system loaded with Psiadia punctulata major metabolites for hypertensive emergencies: effect on hemodynamics and cardiac conductance. Fron Pharmacol. 2021; 12:681070. https://doi.org/10.3389/fphar.2021.681070 DOI: https://doi.org/10.3389/fphar.2021.681070
55. Nestel P, Clifton P, Noakes M. Effects of increasing dietary palmitoleic acid compared with palmitic and oleic acids on plasma lipids of hypercholesterolemic men. J Lipid Res. 1994;35(4):656-662. https://doi:10.1016/S0022-2275(20)41179-4 DOI: https://doi.org/10.1016/S0022-2275(20)41179-4
56. Ashmawy NA, Salem MZ, Mervat EH, Abd El-Kareem MS, El-Shanhorey NA, Mohamed AA, Salem AZ. Antibacterial activity of the bioactive compounds identified in three woody plants against some pathogenic bacteria. Microb Pathog. 2018;121:331-340. https://doi.org/10.1016/j.micpath.2018.05.032 DOI: https://doi.org/10.1016/j.micpath.2018.05.032
57. Sherzai AZ, Sherzai AN, Sherzai D. A systematic review of omega-3 consumption and neuroprotective cognitive outcomes. Am J Lifestyle Med. 2023;17(4):560-88. https://doi.org/10.1177/155982762211171 DOI: https://doi.org/10.1177/15598276221117102
58. Britten-Jones AC, Kamel JT, Roberts LJ, Braat S, Craig JP, MacIsaac RJ, Downie LE. Investigating the neuroprotective effect of oral omega-3 fatty acid supplementation in type 1 diabetes (nPROOFS1): a randomized placebo-controlled trial. Diabet. 2021;70(8):1794-806. https://doi.org/10.2337/db21-0136 DOI: https://doi.org/10.2337/db21-0136
59. Kalogerou M, Ioannou S, Kolovos P, Prokopiou E, Potamiti L, Kyriacou K, Panagiotidis M, Ioannou M, Fella E, Worth EP, Georgiou T. Omega-3 fatty acids promote neuroprotection, decreased apoptosis and reduced glial cell activation in the retina of a mouse model of OPA1-related autosomal dominant optic atrophy. Exp Eye Res. 2022; 215:108901. https://doi.org/10.1016/j.exer.2021.108901 DOI: https://doi.org/10.1016/j.exer.2021.108901
60. Odiase-Omoighe JO, Agoreyo BO. Identification of Bioactive Compounds in Sclerotia Extracts from Pleurotus tuber-regium (Fr.) Sing. using Gas Chromatograph–Mass Spectrometer (GC-MS). Nig. J. Biotech. 2022;38(1):39-50. 10.4314/njb.v38i1.4S DOI: https://doi.org/10.4314/njb.v38i1.4S
61. Usin SG, Daramola OO. Phytochemical analysis and evaluation of ethanol and aqueous extracts of Piliostigma thonningii leaf for in vitro antioxidant activities. Niger J Biochem Mol Biol. 2022;37(1):72-81. e-ISSN: 2659-0042.
62. Olela B, Mbaria J, Wachira T, Moriasi G. Acute oral toxicity and anti-inflammatory and analgesic effects of aqueous and methanolic stem bark extracts of Piliostigma thonningii (Schumach.). Evid Based Complement Alternat Med. 2020;2020:1-10. https://doi.org/10.1155/2020/5651390. DOI: https://doi.org/10.1155/2020/5651390
63. Duenngai K, Promraksa B, Janthamala S, Thanee M, Sirithawat P, Wisungre S, Deechan S, Meechai N, Paratang P, Techasen A. Antioxidant and Anticancer Potentials and Metabolic Profiling of Benjakul, A Thai Herbal Preparation. Trop. J. Nat. Prod. Res. 2024;8(4):6877-83 https://doi.org/10.26538/tjnpr/v8i4.18 DOI: https://doi.org/10.26538/tjnpr/v8i4.18
64. Seyi OM, Lydia FE, Faderera OR. Phytochemicals, toxicity, antioxidant and antimicrobial activities of active secondary metabolites of stem bark of Piliostigma thonningii (Schum) Milne-Redh. Int J Adv Acad Res. 2023;8(5):15-27.
65. Ighodaro OM, Agunbiade SO, Omole JO, Kuti OA. Evaluation of the chemical, nutritional, antimicrobial and antioxidant-vitamin profiles of Piliostigma thonningii leaves. Res J Med Plant. 2012;6(7):537-43. https://scialert.net/abstract/?doi=rjmp.2012.537.543 DOI: https://doi.org/10.3923/rjmp.2012.537.543