Ninety Days Repeated Sub-Chronic Toxicity Study of Ethanol Leaf Extract of Laggera Aurita Linn (Asteracea) In Wistar Rats
Main Article Content
Abstract
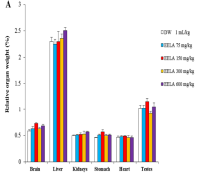
Laggera aurita Linn (Asteraceae) is widely used in Nigeria, Cameroon, Ghana, Senegal, and India for treating malaria, cancer, epilepsy, tuberculosis, and rheumatoid arthritis. This study seeks to establish the safety profile of the ethanol extract of Laggera aurita (EELA). An acute toxicity limit test at 5000 mg/kg was conducted as per OECD 425 and determined to be > 5000 mg/kg. A 90-day oral chronic toxicity study followed, according to OECD 408, involving five groups of ten rats administered the extract at 75, 150, 300, and 600 mg/kg daily, while the control group received 1 ml/kg distilled water. Daily measurements included food and water intake, and body weights were measured weekly. The animals were humanely killed on day 91, and blood samples and vital organs were collected for assays and histopathology. Results indicated no changes in food intake, water intake, body weights, relative organ weights, haematological indices, or reproductive hormones. No elevations of liver transaminase enzymes, bilirubin, or total protein were observed, nor changes in serum creatinine, blood urea nitrogen, or electrolytes. However, a significant (p<0.05) reduction in aspartate transaminase (AST) and urea was observed in the liver and kidney at doses of 150-600 mg/kg compared to control. Additionally, there was a significant (p<0.05) reduction in thyroid hormones and an elevation of cholesterol and low-density lipoproteins at doses of 150-600 mg/kg. The study suggests EELA is generally safe at low doses (75 mg/kg), with the no observed adverse effect level (NOAEL) determined to be 75 mg/kg.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.Kpemissi M, Metowogo K, Melila M, Veerapur VP, Negru M, Taulescu M, Potârniche AV, Suhas DS, Puneeth TA, Vijayakumar S, Eklu-Gadegbeku K, Aklikokou K. Acute and Subchronic Oral Toxicity Assessments of Combretum micranthum (Combretaceae) in Wistar rats. Toxicol Rep. 2020 18(7):162-168. doi: 10.1016/j.toxrep.2020.01.007. PMID: 31993335; PMCID: PMC6976914. DOI: https://doi.org/10.1016/j.toxrep.2020.01.007
2.Oladipupo AR, Alaribe CS, Balogun OT, Akere HT, Ayanda FA, Toye ET, Coker HAB. Investigation of Chemical, Genotoxic and Haematological Properties of Secondary Metabolites From N-Hexane Extract of Olax Subscorpioidea Oliv. (Olacaceae) Leaves. Trp J Nat Prod Res. 2018; 2(12):506-511 DOI: https://doi.org/10.26538/tjnpr/v2i12.3
3.Vaddi ANV Harita, Soumitra Sahana, Sumanta Mondal. Evaluation of Toxicity and Anticonvulsant Activities of Solanum torvum Sw., Fruits. Trop J Nat Prod Res, 2025; 9(2): 613 -627 DOI: https://doi.org/10.26538/tjnpr/v9i2.24
4.Rosidah I, Renggani TN, Firdausi N, Ningsih S, Yunianto P, Permatasari D, Pongtuluran OB, Bahua H, Efendi J, Kusumastuti SA, Nuralih, El Muttaqien, S, Nizar, Kusumaningrum S, Agustini K. Acute and Subchronic Toxicological Study of the Cocktail Extract from Curcuma xanthorrhiza Roxb, Phyllanthus niruri L. and Morinda citrifolia L., J Toxicol 2024; 1-16 https://doi.org/10.1155/2024/9445226 DOI: https://doi.org/10.1155/2024/9445226
5.Abdullah MT, Mohammed M, Abubakar A, Na’imatu AS, Mohammed MS, Mohammed HS, Emmanuel O. Acute, Sub-acute, Sub-chronic and Chronic Toxicity Studies of Four Important Ethnomedicinal Plants In Nigeria 2021; 7(1) 1-12 DOI: https://doi.org/10.1186/s40816-020-00244-2
6.Burkil, HM. The Useful Plants of West Tropical Africa, Vol. 1 (2nd ed). Royal Botanical Gardens, New England, 1985. 452-453.
7.Njan NAM, Saotoing P, Tchouonkeu JC, and Messi J. Effects of Essential Oils of Six Local Plants Used as Insecticide on Adults of Anopheles gambiae. J Ethnopharmacol. 2007; 4(6): 444-450. DOI: https://doi.org/10.3923/je.2007.444.450
8.Okhale SE, Odiniya EO, and Kunle OF. Preliminary Phytochemical and Pharmacogonistical Investigation of Pediatrics Antimalarial Effects of Laggera petrodonta (DC) of Nigerian origin. Ethno Leaf 2010 (4): 457-466.
9.Malami S, Kyari H, Danjuma NM, Ya’u J, and Hussaini IM. Anticonvulsant Properties of Methanol Leaf Extract of Laggera aurita Linn. F (Astraceae). J Ethnopharmacol. 2016; 191(2016): 301-306 DOI: https://doi.org/10.1016/j.jep.2016.06.035
10.Julde SM, Borodo SB, Wada AS, Malam, S, and Bichi LA. Preliminary Phytochemical Screening and Quantitative Analysis of Crude Extract of Laggera aurita Linn (Asteraceae). Rec Chem Sci. 2024; 3(2); 22-27 DOI: https://doi.org/10.33003/frscs_2024_0302/03
11.OECD guideline 425 Acute Oral Toxicity Study- Up and Down Method
12.OECD guideline 408 Repeated Dose 90-Day Oral Toxicity Study In Rodents
13.Mubarak H A, Zezi UA, Anafi SB, Alshargi OY, Mohammed M. Mustapha S, Bala AA, Muhammad S, Julde SM, Wada AS, Jatau AI. Sub‐Acute Toxicity Study on Hydromethanolic Leaves Extract of Combretum Hypopilinum (Combretaceae) Diels in Wistar Rats. Toxicol Res. 2022; 38(4):487-502 DOI: https://doi.org/10.1007/s43188-022-00133-5
14.Amran N, Ermalyanti F, Muhammad F. R. H. Y, Muhammad Z. A. Disi, Yusri S, Muhammad SAS, Hasyrul H. Acute Toxicity Studies of Nutmeg (Myristica fragrans Houtt.) Pulp Ethanol Extractin Balb/c Mice Trop JNat Prod Res, April 2025; 9(4):1539 -1542 DOI: https://doi.org/10.26538/tjnpr/v9i4.23
15.Shehu A. Temidayo O, Zezi AU, and Ahmad A. (2016). Acute Toxicological, Analgesic and Anti-inflammatory Effects of Methanol Extract of Laggera aurita Linn F (compositae) in Mice. Afri J Pharm and Thr. 2016; 5(2): 65-73.
16.Julde S, Kyari H, Malami S. Assessment of Serum Parameters After 28-Day Oral Administration of Methanol Leaf Extract of Lagerra aurita in Wistar Rats. Trop J Nat Prod Res. 2017; 1 (6): 251-254 DOI: https://doi.org/10.26538/tjnpr/v1i6.5
17.Komlan M. D., Aboudoulatif D., Povi L. E., Yendube, T. K., Tchin D., Batomayena B., Kwashie E. G. A 90-Day Oral Toxicity of Hydroethanolic Root Extract of Carissa spinarum in Wistar Rats, J Toxicol. 2021; (1): 1-6 DOI: https://doi.org/10.1155/2021/5570206
18.Ebohon O, Irabor F, Omoregie E.S. Sub-Acute Toxicity Study of Methanol Extract of Tetrorchidium Didymostemon Leaves Using Biochemical Analyses and Gene Expression in Wistar Rats, Heliyon. 2020; 6(2020): 1-9 DOI: https://doi.org/10.1016/j.heliyon.2020.e04313
19.Bailey SA, Zidell RH, Perry R.W. Relationships Between Organ Weight and Body/Brain Weight in the Rat: What Is the Best Analytical Endpoint? Toxicol Path. 2004; 32:448–466. DOI: https://doi.org/10.1080/01926230490465874
20.Nirogi R, Goyal VK, Jana S, Pandey SK, and Gothi A. (2014). What Suits Best for Organ Weight Analysis: Review of Relationship Between Organ Weight and Body/ Brain Weight for Rodent Toxicity Studies, Int J Pharm Sci and Res. 2014; 5(4): 1525-1532
21.Waller DG, Sampson AP. Medical Pharmacology and Therapeutics. Elsevier Philadelphia. 2018; Pp. 64-71.
22.Kaushansky K. Lineage-Specific Hemaopoetic Growth Factors. N Eng J Med. 2006; 354(19): 2034-2045. DOI: https://doi.org/10.1056/NEJMra052706
23.Hervé E, Nomane E, Goze B, Léandre K, Angoué K, Yapo P. Effects of Subacute Oral Administration of Aqueous Extract of Macaranga Barteri Müll Arg (Euphorbiaceae) Leaf on Anthropometric and Haematological Parameters in Rats. Toxicol Res. 2020; 37(1):135-146 DOI: https://doi.org/10.1007/s43188-020-00048-z
24.Kabak M, Çil B, Hocanli I. Relationship Between Leukocyte, Neutrophil, Lymphocyte, Platelet Counts, And Neutrophil to Lymphocyte Ratio and Polymerase Chain Reaction Positivity. Int Immunopharmacol. 2021; 93(2021): 1-6 DOI: https://doi.org/10.1016/j.intimp.2021.107390
25.Joseph T. Opeyemi, Joseph S. Oyepata, Zubairu A. Sabastine, Onyia F. Chukwuebuka. Haematological and Histopathological Effects of Ethanol Extract of Bauhinia variegata (Linn.) on the Brain, Lungs and Spleen of Wistar Rats Trop JNat Prod Res, 2025; 9(4):1672 – 167 DOI: https://doi.org/10.26538/tjnpr/v9i4.41
26.Temple RJ, and Himmel MH. Safety of Newly Approved Drugs: Implications 4 presenting. JAMA. 2002; 287(17): 2273-2275. DOI: https://doi.org/10.1001/jama.287.17.2273
27.Jon Farizal, Zamharira Muslim, Rahmad Abdillah. Acute Oral Toxicity Assessment of Morinda citrifolia L. (Noni) Leaves in Experimental Mice Trop JNat Prod Res, 2024; 8(12): 9586 - 9590 DOI: https://doi.org/10.26538/tjnpr/v8i12.34
28.Almazroo OA, Miah MK, Venkataramanan R. Drug Metabolism in the Liver. Clin Liver Dis. 2017; 21(1):1-20 DOI: https://doi.org/10.1016/j.cld.2016.08.001
29.Farzaei MH, Bayrami Z, Farzaei F, Aneva I, Das SK, Patra JK, Das G, Abdollahi M. Poisoning by Medical Plants. 2020; Arch Iranian Med 23(2):117-127
30.Limdi JK, Hyde GM. Evaluation of Abnormal Liver Function Tests. Postgr Med J. 2003; 79(932): 307-312. DOI: https://doi.org/10.1136/pmj.79.932.307
31.Kpomah ED, Arghoghro EM, Uwakwe A. (2012). Biochemical and Histopathological Changes in Wistar Rats Following Chronic Administration of Di herbal Mixture of Zanthoxylum leprieurii and Piper guineense. J Nat Sci Res. 2012; 2(6): 22-28
32.Rogério S. Chivodze, Atifa I. Sulemane, Isabel Magaia, Vanina N.C. Saete, Saquina C. Rugunate, Hercílio E. Zimila, François Munyemana. Phytochemical screening and Evaluation of Antioxidant and Antimicrobial Activity of Solanum linnaeanum Extracts. Trop JNat Prod Res, 2022; 6(2):194-201
33.Schnellman RG. Toxic Responses of Kidney In: C.D. Klassen (ed), Casarett and Doull’s Toxicology: The Basic Sciences of Poisons, (7th ed), New York McGraw – Hill 2008; 583-608.
34.Oluwatosin A. Dosumu, Adelani I. Bababode, Solomon O. Rotimi, Adio J. Akamo, Oluwatosin O. Omotosho, Latifah O. Sani, Kehinde T. Osinuga, Odunayo A. Taiwo, Oluwafemi P. Owolabi, Oluwafemi A. Ojo. DMBA-Induced Kidney
Dysfunction: Effects of Supplementary Dietary Vitamin K in Rats. Trop JNat Prod Res, 2021; 5(5):917-923 DOI: https://doi.org/10.26538/tjnpr/v5i5.19
35.Anh N. Dang, Wang Yao-Guang, Zhao Xi, Zhang Jing, Hoang N. Nguyen. Effects of Tang Shen 2 Hao Fang, a Traditional Chinese Medicine, on Diabetic Nephropathy in Sprague–Dawley Rats Trop JNat Prod Res, 2021;5(10):1828-1834 DOI: https://doi.org/10.26538/tjnpr/v5i10.20
36.Edmund L, David J. Kidney Function Tests. In: Carl AB, Edward R, David E. T. (eds). Textbook of Clinical Chemistry and Molecular Diagnostics. 4th Ed. New Delhi: Elsevier Inc.; 2006. 797-808.
37.Houda Z. Megherbi, Sara Aouissat, Farida O. Boukortt. Effect of Casein Hydrolysates and Zinc on Insulin Resistance, Renal Function, Pancreatic and Kidney Histopathology in Diabetic Rats, Trop JNat Prod Res, 2025; 9(4):1441 – 1448 DOI: https://doi.org/10.26538/tjnpr/v9i4.9
38.Lippi G, Plebani M. Current Trends and Future Projections in the Clinical Laboratory Test Market: Implications for Resource Management and Strategic Planning. Clin Chem Lab Med 2025;63(4): e91-e93. https://doi.org/10.1515/cclm-2024-1424 DOI: https://doi.org/10.1515/cclm-2024-1424
39.Enaohwo T Mamerhi, Orororo C Osuvwe, Jaiyeoba-ojigho E Jennifer, Dunkwu C Cyril, Enyi C Kingsley, Oghaimeh O Grace, Mode, Joan. Justicia carnea Extract Mitigates TNBS-Induced Liver Inflammation by Ameliorating Oxidative Stress, Improving Liver Function Indices and Normalizing NF-κb/Caspase 3 Expression. Trop J Nat Prod Res 2025; 9(4): 1574 -1583 DOI: https://doi.org/10.26538/tjnpr/v9i4.29
40.Espinoza SE, Guo H, Fedarko N, Fried PL, Xue QL, Leng S, Beamer B, Walston JD. Glutathione Peroxidase Enzyme Activity in Ageing, J. Gerontol Series A, Bioland Med Sci. 2008; 63(5): 505-509. DOI: https://doi.org/10.1093/gerona/63.5.505
41.Chu Z, Fang L, Xiang Y, Ding Y. Research Progress on Cholesterol Metabolism and Tumour Therapy. Discov Onc 2025; (16):647. https://doi.org/10.1007/s12672-025-02430-5 DOI: https://doi.org/10.1007/s12672-025-02430-5
42.Chapman, M.J., Ginsberg, H.N., Amarenco, P., Andreotti, F., Boren, J., Catapano, Al., Descamps, O.S., Fisher, E., Kovanen, P.T., Lesnik, J.P., Masana, L., Reiner, Z., Taskinen, M.R., Tokgozolu, A.H., And Watts, G.F. Triglyceride Rich Lipoproteins and High-Density Lipoprotein n Patients at High Risk of Cardiovascular Disease: Evidence and Guidance for Management. EHJ. 2011; 32(11):1345-1361 DOI: https://doi.org/10.1016/S1567-5688(11)70033-2
43.Rizos, C.V., Elisaf, M.S., and Liberopoulos, E.N. Effects of Thyroid Dysfunction on Lipid Profile. Open Cardiovasc Med J. 2011; (5): 76-84 DOI: https://doi.org/10.2174/1874192401105010076
44.Mandava, S., and Mandava, N.B. Endocrine Disrupting Chemicals and the Effects They Play in Plant Regulators J. Endocr Disord. 2021; 7(1): 1-6 DOI: https://doi.org/10.26420/jendocrdisord.2021.1046
45.Mattison D. R. The Mechanisms of Action of Reproductive Toxins. Am J Ind Med, 1983; 4(1-2): 65–79. DOI: https://doi.org/10.1002/ajim.1983.4.1-2.65
46.Smith C. G. Reproductive Toxicity: Hypothalamic-Pituitary Mechanisms. Ame J Ind Med. 1983; 4(1-2): 107–112. DOI: https://doi.org/10.1002/ajim.1983.4.1-2.107
47.Kuchi Manjeera, Raja Sundararajan. Toxicity Studies of Abutilon crispum and Indigofera prostrata Whole Plants on Wistar Rats, Trop JNat Prod Res, 2024; 8(11): 9073 – 9078 DOI: https://doi.org/10.26538/tjnpr/v8i11.15
48.Chinnappan, S., George, A., Ashok, G., & Choudhary, Y. K. Effect of Herbal Extract Eurycoma Longifolia (Physta) On Female Reproductive Hormones and Bone Biochemical Markers: An Ovariectomised Rat Model Study. BMC Compl Med and Ther. 2020; 20(31): 1-12 DOI: https://doi.org/10.1186/s12906-020-2814-z
49.Juliet N. Olayinka, Abigail M. Akhigbemen, Emmar E. Okpakpor, Dickson O. Uwaya, Gerald I. Eze, Raymond I. Ozolua. Toxicological Evaluation of the Aqueous Leaf Extract of Blighia Sapida K.D. Koenig (Sapindaceae) in Rodents. Trop JNat Prod Res, 2023; 7(12) 5690-5698 DOI: https://doi.org/10.26538/tjnpr/v7i12.48