Isolation and Characterisation of Bioactive Principles from Sapwood of Pterocarpus santalinoides
Main Article Content
Abstract
Pterocarpus santalinoides is a medicinal plant widely employed in traditional Nigerian medicine for managing diverse health conditions. The present study focuses on the isolation and characterisation of biologically active compounds from the sapwood of Pterocarpus santalinoides.
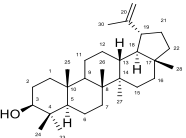
. Sequential extraction was carried out using n-hexane, ethyl acetate, and methanol, followed by purification of the ethyl acetate extract through column chromatography. Thin Layer Chromatography (TLC) was employed for fraction monitoring, while the structures of isolated compounds were elucidated using ¹H, ¹³C, and 2D NMR techniques, including HMBC, HSQC, and COSY, in comparison with existing literature. The identified compounds included lupeol, a mixture of β-sitosterol and stigmasterol, a 1,2-diacylglycerolipid containing polyunsaturated ω-6 and ω-3 acyl chains, a diacylglycerol with one polyunsaturated and two saturated/monounsaturated chains, and oleanolic acid. Crude extracts of Pterocarpus santalinoides, including ethyl acetate, methanol, and hexane, exhibited significant antimicrobial activity against both Gram-positive and Gram-negative microorganisms. Notably, fraction EPs14 demonstrated superior efficacy, with inhibition zones of 23 mm to 27 mm against various fungi and bacteria. EPs26 and EPs36 showed the highest inhibition zones of 29 mm and 30 mm, respectively, against Phaeolus schweinitzii and Coniophora puteana. Minimum inhibitory concentrations (MIC) for these fractions were as low as 6.25 µg/mL. These findings support the traditional use of P. santalinoides in the treatment of diarrhoea, placentitis, skin infections, and gastrointestinal disorders, marking the first report of these bioactive compounds from the plant’s sapwood.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.Devi S, Gupta AK, Singh, M. Ethno-Medicinal Use of Plants Belonging to Families Fabaceae and Solanaceae, Hamirpur District (H.D). Int. J. Sci. Res. 2012; 2(3): 1-4.
2.Nwokorie CC, Nwachukwu NC, Ezeanokete CC, Ike CC. The Phytochemical and Antimicrobial Analysis of Pterocarpus santalinoides Plants. Asia-Pac. J. Sci. Technol. 2015; 6(5): 1411-1418.
3.Odeh IC, Tor-Anyiin TA. Phytochemical and Antimicrobial Evaluation of Leaf-Extracts of Pterocarpus santalinoides. European J. Med. Plants. 2014; 4: 6-12 ISSN: 2231-0894. DOI: https://doi.org/10.9734/EJMP/2014/5455
4.Aja PM, Ugwu C, Onuola MC, Ogbu NP, Nweke OL, Ali IA. Comparative Proximate Analysis of Pterocarpus santalinoides and Ficus carpensis Leaves from Abakiliki, Ebonyi State Nigeria. Caribb. J. Sci. 2016; 4: 877-881. ISSN 07993757.
5.Rasool HBA. Medicinal Plants (Importance and Uses). Anal. Chim. Acta. 2012; 3: 10, Doi: 10.4172/2153-2435.1000e139. DOI: https://doi.org/10.4172/2153-2435.1000e139
6.Okpo SO, Ching SF, Ekeleme IC. Evaluation of the Anti-Diarrhoea Activity of the Aqueous Extract from Leaves of Pterocarpus santalinoides. Res. J. Pharm. Biol. Chem. Sci. 2011; 2(3): 590-597.
7.Igoli JO. Traditional medicine practice amongst the Igede people of Nigeria, part II. Int. J. Biotechnol. 2005;4(4): 408-412 DOI: https://doi.org/10.4314/ajtcam.v2i2.31112
8.Odeh IC, Tor-Anyiin TA, Igoli JO, Anyam JV. In-vitro antimicrobial properties of friedelan-3-one from Pterocarpus santalinoides L-Herit, ex Dc. Afr. J. Biotechnol. 2016; 15(14), 531-538. DOI: https://doi.org/10.5897/AJB2015.15091
9.Igoli OJ, Gray IA. Friedelanone and Other triterpenoids from Hymenocardia acida, Int. J. Phys. Sci. 2008; 3(6): 156-158.
10.Rodríguez-Melcón C, Alonso-Calleja C, García-Fernández C, Carballo J, Capita R. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) for Twelve Antimicrobials (Biocides and Antibiotics) in Eight Strains of Listeria monocytogenes. Biol. 2022; 11(46): 1-16. https://doi.org/ 10.3390/biology11010046. DOI: https://doi.org/10.3390/biology11010046
11.Ipav SS, Igoli JO, Tor-Anyiin TA, Anyam JV. Isolation and Characterisation of Lupeol and Lupeol Acetate from Propolis Obtained from Benue State. J. Chem. Soc. Nigeria. 2022; 47(1): 152-159. DOI: https://doi.org/10.46602/jcsn.v47i1.708
12.Burns D, Reynolds WF, Buchanan G, Reese PB, Enriquez RG. Assignment of 1H and 13C Spectra and Investigation of Hindered Side-Chain Rotation in Lupeol Derivatives. Magn. Reson. Chem. 2000; 38(7): 488-493. Doi: 10.1002/1097-458x(200007)38:7<488::aid-mrc704>3.0.co;2-g. DOI: https://doi.org/10.1002/1097-458X(200007)38:7<488::AID-MRC704>3.3.CO;2-7
13.Ododo MM, Choudhury MK, Dekebo AH. Structure Elucidation of β-Sitosterol with Antibacterial Activity from the Root Bark of Malva parviflora. Springerplus. 2016; 5(1210): 1-11. Doi: 10.1186/540064-016-2894-x. DOI: https://doi.org/10.1186/s40064-016-2894-x
14.Luhata LP, Munkombwe NM. Isolation and Characterisation of Stigmasterol and β-Sitosterol from Odontonemastrictum (Acanthaceae). J. Innov. Pharm. Biol. Sci. 2015; 2(1): 88-96 Doi:10.1314012G.2.1.3689.7365
15.Nnamonu LA, Tor-Anyiin TA, Ugbenyo NO, Anyam JV. Isolation and Characterisation of α-Amyrin from Stem Bark of Ficus exasperate (Vahl). Biotechnol. J. Int. 2016;16(4): 1-7 DOI: https://doi.org/10.9734/BJI/2016/29737
16.Okoye NN, Ajagbaku DL, Okeke HN, Ilodigwe EE, Nworu CS, Okoye EBC. Beta-Amyrin and Alpha-Amyrin Acetate Isolated from the Stem Bark of Alstonia Boonei Display Profound Anti-Inflammatory Activity. Pharm Biol. 2014; 52(11): 1478-1486. Doi: 10.3109/13880209.2014.898078. DOI: https://doi.org/10.3109/13880209.2014.898078
17.Ipav SS, Igoli JO, Tor-Anyiin TA, Anyam JV. Isolation and Characterisation of Alpha and Beta Amyrins from Propolis Obtained from Benue State. J. Chem. Soc. Nig. 2022; 47(2): 250-261. DOI: https://doi.org/10.46602/jcsn.v47i2.723
18.Patra A, Mitra AK, Ghosh S, Ghosh A, Barua AK. Carbon 13 NMR Spectroscopy of Some Pentacyclic Triterpenoids. Org. Magn. Reson. 1981; 15(4): 399-408. DOI: https://doi.org/10.1002/mrc.1270150415
19.Gwandu UZ, Dangoggo SM, Faruk UZ, Halilu EM, Yusuf AJ, Mailafia MM. Isolation and Characterisation of Oleanolic Acid Benzoate from Ethylacetate Leaves Extracts of Vernonia ambigua (Kotchi Ex Peyr). J. Chem. Soc. Nigeria. 2020; 45(1): 871-876. DOI: https://doi.org/10.46602/jcsn.v45i5.518
20.Nieva-Echevarría B, Goicoechea E, Manzanos MJ, Guillén MD. Usefulness of 1H NMR in assessing the extent of lipid digestion. Food Chem. 2015 Jul 15;179:182-90. DOI: https://doi.org/10.1016/j.foodchem.2015.01.104
21.Hostettmann K, Hostettmann M, Marston A. Preparative chromatography techniques: Applications in Natural Product Isolation. Springer Berlin, Heidelberg. 1986. Pp230-240 DOI: https://doi.org/10.1007/978-3-662-02492-8