Antihypertensive and Vasorelaxant Effects of Cistanche phelypaea Leaves through Inhibition of Receptor-Operated Calcium Channels in Rats
Main Article Content
Abstract
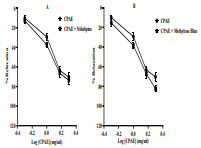
Cistanche phelypaea (family: Orobanchaceae) is a medicinal plant traditionally used for its vasorelaxant, diuretic, and sedative properties, highlighting its potential relevance in the management of hypertension. The present study aimed to evaluate the antihypertensive and vasorelaxant effects of Cistanche phelypaea leaves and its impact on angiotensin-converting enzyme 2 (ACE-2). The aqueous leaf extract of Cistanche phelypaea (CPAE) was prepared and its antihypertensive effect (200 mg/kg) was examined using a hypertensive rat model induced by oral administration of L-NAME (60 mg/kg bw), additionally, we assessed its vasorelaxant potential and its impact on the stimulation or inhibition of ACE-2 in isolated rat thoracic aorta. The results indicate that CPAE significantly lowered systolic (p<0.0001), diastolic (p<0.01), and mean arterial pressure (p<0.01) without affecting normotensive rats. The data showed that CPAE mediates its antihypertensive effect via vasodilatory activity. Furthermore, the vasorelaxant capacity of CPAE seems to be mediated through receptor-operated calcium channels (ROCCs). However, CPAE had no effect on ACE-2. In conclusion, the study demonstrates that the aqueous leaf extract of Cistanche phelypaea possesses a significant antihypertensive and vasorelaxant effects, primarily through the inhibition of Ca2+ entry.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.Mittal BV, Singh AK. Hypertension in the developing world: challenges and opportunities. Am J Kidney Dis. 2010; 55(3):590–598. Doi:10.1053/j.ajkd.2009.06.044 DOI: https://doi.org/10.1053/j.ajkd.2009.06.044
2.Singh P, Mishra A, Singh P, Goswami S, Singh A, Tiwari KD. Hypertension and herbal plant for its treatment: a review. Indian J Res Pharm Biotechnol. 2015;3(5):358. Doi :10.34172/apb.2021.090
3.Bopda OSM, Longo F, Bella TN, Edzah PMO, Taïwe GS, Bilanda DC, Dimo T. Antihypertensive activities of the aqueous extract of Kalanchoe pinnata (Crassulaceae) in high salt-loaded rats. J Ethnopharmacol. 2014;153(2):400–407. Doi: 10.1016/j.jep.2014.02.041 DOI: https://doi.org/10.1016/j.jep.2014.02.041
4.Eddouks M, Maghrani M, Lemhadri A, Ouahidi ML, Jouad H. Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet). J Ethnopharmacol. 2002;82(2–3):97–103. Doi:10.1016/S0378-8741(02)00164-2 DOI: https://doi.org/10.1016/S0378-8741(02)00164-2
5.Jouad H, Haloui M, Rhiouani H, El Hilaly J, Eddouks M. Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North centre region of Morocco (Fez–Boulemane). J Ethnopharmacol. 2001;77(2–3):175–182. Doi:10.1016/S0378-8741(01)00289-6 DOI: https://doi.org/10.1016/S0378-8741(01)00289-6
6.Ziyyat A, Legssyer A, Mekhfi H, Dassouli A, Serhrouchni M, Benjelloun W. Phytotherapy of hypertension and diabetes in oriental Morocco. J Ethnopharmacol. 1997;58(1):45–54. Doi:10.1016/S0378-8741(97)00077-9 DOI: https://doi.org/10.1016/S0378-8741(97)00077-9
7.Lv HN, Zeng KW, Song YL, Jiang Y, Tu PF. Phytochemical and pharmacological overview of Cistanche species. Recent Adv Polyphenol Res. 2017:313–341. Doi:10.1002/9781118883303.ch14 DOI: https://doi.org/10.1002/9781118883303.ch14
8.Li Z, Lin H, Gu L, Gao J, Tzeng CM. Herba Cistanche (Rou Cong-Rong): one of the best pharmaceutical gifts of traditional Chinese medicine. Front Pharmacol. 2016;7:41. Doi:10.3389/fphar.2016.00041 DOI: https://doi.org/10.3389/fphar.2016.00041
9.Trampetti F, Pereira C, Rodrigues MJ, Celaj O, D’Abrosca B, Zengin G, Mollica A, Stefanucci A, Custódio L. Exploring the halophyte Cistanche phelypaea (L.) Cout as a source of health promoting products: in vitro antioxidant and enzyme inhibitory properties, metabolomic profile and computational studies. J Pharm Biomed Anal. 2019;165:119–128. Doi:10.1016/j.jpba.2018.11.053 DOI: https://doi.org/10.1016/j.jpba.2018.11.053
10.Bouzitouna A, Ouali K, Djeddi S. Protective effects of Cistanche tinctoria aqueous extract on blood glucose and antioxidant defense system of pancreatic β-cells in experimental diabetes in rats. Int J Pharm Sci Rev Res. 2015;32(2):243–249.
11.IUCN Centre for Mediterranean Cooperation. A guide to the medicinal plants of North Africa. Malaga, Spain: IUCN CMC; 2005. 256 p.
12.Lakhdari W, Dehliz A, Acheuk F, Mlik R, Hammi H, Doumandji-Mitiche B, Gheriani S, Berrekbia M, Guermit K, Chergui S. Ethnobotanical study of some plants used in traditional medicine in the region of Oued Righ (Algerian Sahara). 2016.
13.Bouadid I, Amssayef A, Eddouks M. Study of the antihypertensive effect of Laurus nobilis in rats. Cardiovasc Hematol Agents Med Chem. 2023;21(1):42–54. Doi:10.2174/1871525720666220512154041 DOI: https://doi.org/10.2174/1871525720666220512154041
14.Ajebli M, Eddouks M. Antihypertensive activity of Petroselinum crispum through inhibition of vascular calcium channels in rats. J Ethnopharmacol. 2019;242:112039. Doi:10.1016/j.jep.2019.112039 DOI: https://doi.org/10.1016/j.jep.2019.112039
15.Amssayef A, Eddouks M. Aqueous extract of Matricaria pubescens exhibits antihypertensive activity in L-NAME-induced hypertensive rats through its vasorelaxant effect. Cardiovasc Hematol Agents Med Chem. 2019;17(2):135–143. Doi:10.2174/1871525717666191007151413 DOI: https://doi.org/10.2174/1871525717666191007151413
16.Amssayef A, Bouadid I, El-Haidani A, Eddouks M. Antihypertensive and vasorelaxant effects of Rumex vesicarius (L.) through receptor-operated calcium channels in hypertensive rats. Cardiovasc Hematol Disord Drug Targets. 2022;22(1):67–82. Doi:10.2174/1871529X22666220531110308 DOI: https://doi.org/10.2174/1871529X22666220531110308
17.Zhang N. Study on the regulation effect of polysaccharides from Cistanche deserticola on endocrine function of menopausal hypertensive rats with dryness syndrome. J Trad Chin Med. 2018;46:65–69. Doi:10.19664/j.cnki.1002-2392.180016
18.Duff F, Greenfield AD, Shepherd JT, Thompson ID, Whelan RF. The response to vasodilator substances of the blood vessels in fingers immersed in cold water. J Physiol. 1953;121(1):46. Doi:10.1113/jphysiol.1953.sp004929 DOI: https://doi.org/10.1113/jphysiol.1953.sp004929
19.Martinsen A, Dessy C, Morel N. Regulation of calcium channels in smooth muscle: new insights into the role of myosin light chain kinase. Channels. 2014;8(5):402–413. Doi:10.4161/19336950.2014.950537 DOI: https://doi.org/10.4161/19336950.2014.950537
20.Thorneloe KS, Nelson MT. Ion channels in smooth muscle: regulators of intracellular calcium and contractility. Can J Physiol Pharmacol. 2005;83(3):215–242. Doi:10.1139/y05-016 DOI: https://doi.org/10.1139/y05-016
21.McCarron JG, Bradley KN, MacMillan D, Muir TC. Sarcolemma agonist-induced interactions between InsP3 and ryanodine receptors in Ca2+ oscillations and waves in smooth muscle. Biochem Soc Trans. 2003;31(5):920–924. Doi:10.1042/bst0310920 DOI: https://doi.org/10.1042/bst0310920
22.Ratz PH, Berg KM, Urban NH, Miner AS. Regulation of smooth muscle calcium sensitivity: KCl as a calcium-sensitizing stimulus. Am J Physiol Cell Physiol. 2005;288(4):C769–C783. Doi:10.1152/ajpcell.00529.2004 DOI: https://doi.org/10.1152/ajpcell.00529.2004
23.Yoshikawa M, Matsuda H, Morikawa T, Xie H, Nakamura S, Muraoka O. Phenylethanoid oligoglycosides and acylated oligosugars with vasorelaxant activity from Cistanche tubulosa. Bioorg Med Chem. 2006;14(22):7468–7475. Doi:10.1016/j.bmc.2006.07.018 DOI: https://doi.org/10.1016/j.bmc.2006.07.018
24.Owolabi OA, Adegunloye BJ, Ajagbona OP, Sofola OA, Obiefuna PC. Mechanism of relaxant effect mediated by an aqueous extract of Hibiscus sabdariffa petals in isolated rat aorta. Int J Pharmacogn. 1995;33(3):210–214. Doi:10.3109/13880209509065365 DOI: https://doi.org/10.3109/13880209509065365
25.Burrell LM, Johnston CI, Tikellis C, Cooper ME. ACE2, a new regulator of the renin–angiotensin system. Trends Endocrinol Metab. 2004;15(4):166–169. Doi:10.1016/j.tem.2004.03.001 DOI: https://doi.org/10.1016/j.tem.2004.03.001
26.Ahmad I, Pawara R, Surana S, Patel H. The repurposed ACE2 inhibitors: SARS-CoV-2 entry blockers of COVID-19. Top Curr Chem. 2021;379:1–49. Doi:10.1007/s41061-021-00353-7 DOI: https://doi.org/10.1007/s41061-021-00353-7
27.Dabaghian F, Khanavi M, Zarshenas MM. Bioactive compounds with possible inhibitory activity of angiotensin-converting enzyme II: a gate to manage and prevent COVID-19. Med Hypotheses. 2020;143:109841. Doi:10.1016/j.mehy.2020.109841 DOI: https://doi.org/10.1016/j.mehy.2020.109841
28.Zheng YY, Ma YT, Zhang JY, Xie X. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17(5):259–260. Doi:10.1038/s41569-020-0360-5 DOI: https://doi.org/10.1038/s41569-020-0360-5
29.De Maria ML, Araújo LD, Fraga-Silva RA, Pereira LAS, Ribeiro HJ, Menezes GB, Shenoy V, Raizada MK, Ferreira AJ. Anti-hypertensive effects of diminazene aceturate: an angiotensin-converting enzyme 2 activator in rats. Protein Pept Lett. 2016;23(1):9–16. Doi:10.2174/0929866522666151013130550 DOI: https://doi.org/10.2174/0929866522666151013130550
30.Amssayef A, Bouadid I, Eddouks M. Vitamin C inhibits angiotensin-converting enzyme 2 in isolated rat aortic ring. Cardiovasc Hematol Disord Drug Targets. 2021;21(4):235–242. Doi:10.2174/1871529X21666211214153308 DOI: https://doi.org/10.2174/1871529X21666211214153308
31.Sartório CL, Pimentel EB, Dos Santos RL, Rouver WN, Mill JG. Acute hypotensive effect of diminazene aceturate in spontaneously hypertensive rats: role of NO and Mas receptor. Clin Exp Pharmacol Physiol. 2020;47(10):1723–1730. Doi:10.1111/1440-1681.13368 DOI: https://doi.org/10.1111/1440-1681.13368