Response Surface Methodology-Driven Optimization of HPLC for the Determination of Caffeine, Chlorogenic Acid, and Caffeic Acid in Coffee
Main Article Content
Abstract
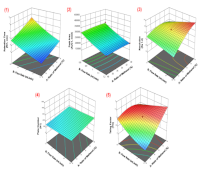
Most studies on coffee bioactive compounds have focused on single targets and relied on trial-and-error optimization. This study aims to develop a high-performance liquid chromatographic (HPLC) method optimized by response surface methodology using a central composite design (RSM-CCD), and a design of experiments (DoE) approach for the simultaneous quantification of caffeine (CAF), chlorogenic acid (CGA), and caffeic acid (CA) in roasted coffee samples. Separation was performed on a Luna C18 (2) column (250 × 4.6 mm; 5 μm). Three factors – methanol ratio, flow rate, and methanol gradient were investigated and optimized through 17 CCD runs, evaluating retention time, peak area, resolution, tailing factor, and theoretical plates. The optimum condition of 31.6% methanol, 0.81 mL/min flow rate, and 1%/min gradient enabled the simultaneous elution and separation of CGA, CAF, and CA at a retention time of 6.325, 7.444, and 9.326 minutes, respectively, and over a 12-minute chromatographic runtime. Validation according to International Council for Harmonization (ICH) Q2 (R1) guidelines confirmed excellent linearity (R² ≥ 0.998), LOD/LOQ (ppm) of 0.1718/0.5207 (CGA), 0.1773/0.5373 (CAF), and 0.0813/0.2464 (CA), with precision below 2% and recovery between 94.05% and 112.42%. Robusta levels (mg/g) were CGA (10.083 ± 0.229), CAF (18.838 ± 0.238), and CA (2.067 ± 0.044) while Arabica levels (mg/g) were CGA (16.641 ± 0.211), CAF (9.916 ± 0.157), and CA (2.178 ± 0.041). The RSM-driven HPLC successfully demonstrated efficient factor interaction modeling and robustness improvement. Consequently, the developed DoE-based method provides a rapid, selective, and reliable method for coffee quality evaluation and authenticity assessment.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.Aborziza M, Amalia R, Zuhrotun A, Ikram NKK, Novitasari D, Muchtaridi M. Coffee bean and its chemical constituent caffeine and chlorogenic acid as promising chemoprevention agents: updated biological studies against cancer cells. Molecules. 2024; 29(14). https://doi.org/10.3390/molecules29143302
2.Socała K, Szopa A, Serefko A, Poleszak E, Wlaź P. Neuroprotective effects of coffee bioactive compounds: a review. Int. J Mol. Sci. 2021; 22(1):1–64. https://doi.org/10.3390/ijms22010107
3.Mirzaei F, Agbaria L, Bhatnagar K, Sirimanne N, Omar A’amar N, Jindal V. Coffee and Alzheimer’s disease. In: Prog Brain Res. Elsevier B.V.; 2024. p. 21–55. https://doi.org/10.1016/bs.pbr.2024.06.002
4.Xiao J, Sarker SD, Asakawa Y. Handbook of Dietary Phytochemicals. 2021. https://doi.org/10.1007/978-981-15-4148-3
5.Ontawong A, Duangjai A, Vaddhanaphuti CS, Amornlerdpison D, Pengnet S, Kamkaew N. Chlorogenic acid rich in coffee pulp extract suppresses inflammatory status by inhibiting the p38, MAPK, and NF-κB pathways. Heliyon. 2023; 9(3). https://doi.org/10.1016/j.heliyon.2023.e13917
6.Yeager SE, Batali ME, Guinard JX, Ristenpart WD. Acids in coffee: a review of sensory measurements and meta-analysis of chemical composition. Crit. Rev Food Sci. Nutr. 2023; 63(8):1010–1036. https://doi.org/10.1080/10408398.2021.1957767
7.Jung S, Gu S, Lee SH, Jeong Y. Effect of roasting degree on the antioxidant properties of espresso and drip coffee extracted from Coffea arabica cv. Java. Appl. Sci. (Basel). 2021; 11(15). https://doi.org/10.3390/app11157025
8.Maity S, Kinra M, Nampoothiri M, Arora D, Pai KSR, Mudgal J. Caffeic acid, a dietary polyphenol, as a promising candidate for combination therapy. Chem. Pap. 2022; 76(3):1271–1283. https://doi.org/10.1007/s11696-021-01947-7
9.Portela C da S, Almeida IF de, Mori ALB, Yamashita F, Benassi M de T. Brewing conditions impact on the composition and characteristics of cold brew Arabica and Robusta coffee beverages. LWT. 2021; 143. https://doi.org/10.1016/j.lwt.2021.111090
10.Heo J, Adhikari K, Choi KS, Lee J. Analysis of caffeine, chlorogenic acid, trigonelline, and volatile compounds in cold brew coffee using high-performance liquid chromatography and solid-phase microextraction—gas chromatography–mass spectrometry. Foods. 2020; 9(12). https://doi.org/10.3390/foods9121746
11.Awwad S, Issa R, Alnsour L, Albals D, Al-Momani I. Quantification of caffeine and chlorogenic acid in green and roasted coffee samples using HPLC-DAD and evaluation of the effect of degree of roasting on their levels. Molecules. 2021; 26(24). https://doi.org/10.3390/molecules26247502
12.Wale K, Tolessa K, Atlabachew M, Mehari B, Alemayehu M, Mengistu DA, Abate D, Engdaw TA, Moges GM. Level of caffeine, trigonelline and chlorogenic acids in green coffee (Coffea arabica L.) beans from Amhara region, Ethiopia. J Agric. Food Res. 2024; 16. https://doi.org/10.1016/j.jafr.2024.101082
13.Brzezicha J, Błażejewicz D, Brzezińska J, Grembecka M. Green coffee vs dietary supplements: a comparative analysis of bioactive compounds and antioxidant activity. Food Chem. Toxicol. 2021; 155. https://doi.org/10.1016/j.fct.2021.112377
14.Klikarová J, Česlová L. Targeted and non-targeted HPLC analysis of coffee-based products as effective tools for evaluating the coffee authenticity. Molecules. 2022; 27(21). https://doi.org/10.3390/molecules27217419
15.Santanatoglia A, Angeloni S, Fiorito M, Fioretti L, Ricciutelli M, Sagratini G, Torregiani E, Mastrogiacomo D, Cortese M, Angeloni C, Caprioli G. Development of new analytical methods for the quantification of organic acids, chlorogenic acids and caffeine in espresso coffee by using solid-phase extraction (SPE) and high-performance liquid chromatography–diode array detector (HPLC-DAD). J Food Compos Anal. 2024; 125. https://doi.org/10.1016/j.jfca.2023.105732
16.Blinova IP, Oleinits EY, Salasina YY, Deineka VI, Anh VTN, Anh N Van. Simultaneous determination of chlorogenic acids and caffeine by reversed-phase HPLC. ChemChemTech. 2023; 66(2):45–52. https://doi.org/10.6060/ivkkt.20236602.6711
17.Himri C, Belkhiri C, Azizi SE, Loukili EH, Rahhou I, Legssyer M, Legssyer B. A Comparative Study of Phenolic Compounds, and the Antioxidant Properties of Arabica Coffee Beans (Coffea arabica) and Robusta Coffee Beans (Coffea canephora) and Their By-Products. Trop J Nat Prod Res. 2025; 9(8): 3568–3580 https://doi.org/10.26538/tjnpr/v9i8.17
18.Najmi A, Rehman Z ur, Zoghebi K, Alhazmi HA, Albratty MM, Haroobi QYH, Alhazmi AA, Ahsan W. Central composite design (CCD) approach to develop HPLC method for caffeine: application to coffee samples analysis of Jazan region, Saudi Arabia. J Saudi Chem. Soc. 2024; 28(1). https://doi.org/10.1016/j.jscs.2023.101772
19.da Silva WB, Rocha LM, Moura LDG, Soares MS, da Silva SA, Moreira DB, Nunes AAP, Silva EF, Lima RG, Silva JF, Oliveira LS. Optimization of methodology for simultaneous quantification of trigonelline, 5-caffeoylquinic acid, and caffeine in green and roasted coffee extracts by HPLC. ACS Omega. 2025; 10(35):40304–40312. https://doi.org/10.1021/acsomega.5c05526
20.Strieder MM, Sanches VL, Rostagno MA. Simultaneous extraction, separation, and analysis of 5-caffeoylquinic acid and caffeine from coffee co-product by PLE-SPE × HPLC-PDA two-dimensional system. Food Res Int. 2024; 175. https://doi.org/10.1016/j.foodres.2023.113690
21.da Silva WB, Rocha LM, Soares MS, Good God PIV, da Silva SA, Moreira DB, Nunes AAP, Silva EF, Lima RG, Silva JF, Oliveira LS. Validation of methodology for quantifying caffeic and ferulic acids in raw and roasted coffee extracts by high-performance liquid chromatography. J (Basel). 2025; 8(1):8. https://doi.org/10.3390/j8010008
22.Kasetti K, Bandhakavi S. Stability indicating simultaneous quantification of chlorogenic acid and berberine in homeopathic polyherbal formulation by AQbD-based HPLC. J Young Pharm. 2025; 17(2):319–328. https://doi.org/10.5530/jyp.20251556
23.Mystkowska I, Dmitrowicz A, Sijko-Szpáńska M. Quantitative analysis of caffeine in roasted coffee: a comparison of brewing methods. Appl. Sci. (Basel). 2024; 14:11395. https://doi.org/10.3390/app142311395
24.Santiago WD, Teixeira AR, Santiago J de A, Lopes ACA, Brandão RM, Caetano AR, Morais CSB, Silva HF, Souza Filho MSM. Development and validation of chromatographic methods to quantify organic compounds in green coffee (Coffea arabica) beans. Aust. J Crop Sci. 2020; 14(8):1275–1282. https://doi.org/10.21475/ajcs.20.14.08.p2433
25.Almeida ERV, Melo AS, Lima AS, Lemos VA, Oliveira GS, Cletche CF, Silva JUN, Santos Neto JH, Nóbrega JA. A review of the use of central composite design in the optimization of procedures aiming at food chemical analysis. Food Chem. 2025; 480. https://doi.org/10.1016/j.foodchem.2025.143849
26.Narukulla S, Bogadi S, Tallapaneni V, Sanapalli BKR, Sanju S, Khan AA, Mukhthiyar M. Comparative study between the full factorial, Box–Behnken, and central composite designs in the optimization of metronidazole immediate-release tablet. Microchem J. 2024; 207. https://doi.org/10.1016/j.microc.2024.111875
27.International Council for Harmonisation. Validation of Analytical Procedures: Text and Methodology Q2(R1). 2005. https://database.ich.org/sites/default/files/Q2%28R1%29%20Guideline.pdf
28.Kraber S. Keys to Analyzing a Response Surface Design. 2021 [accessed 2025 Sep 26]. https://cdn.statease.com/media/public/documents/Keys_to_Analyzing_an_RSM_Design.pdf
29.Anusi MO, Menkiti MC, Ikeuba AI, Njoku CN, Iloegbunam CE, Nnamani CJ, Okeoma KC, Okoronkwo AE, Nwokoye GC, Ezenetune CN, Iwuchukwu UC. Comparative analysis of the response surface methodology (RSM) and artificial neural network (ANN) modelling for the removal of diclofenac potassium from synthesized pharmaceutical wastewater using a palm sheath fiber nano-filtration membrane and optimization. Curr Res Green Sustain Chem. 2025; 11. https://doi.org/10.1016/j.crgsc.2025.100466
30.Kim J, Kim DG, Ryu KH. Enhancing response surface methodology through coefficient clipping based on prior knowledge. Processes. 2023; 11(12). https://doi.org/10.3390/pr11123392
31.U.S. Food and Drug Administration. Center for Drug Evaluation and Research (CDER) Reviewer Guidance: Validation of Chromatographic Methods. 1994 [accessed 2025 Sep 30]. https://www.gmp-compliance.org/files/guidemgr/1-6-13.pdf
32.Chan CC, Lam H, Lee YC, Zhang XM. Analytical Method Validation and Instrument Performance Verification. 2004. https://doi.org/10.1002/0471463728
33.González GA, Herrador ÁM. A practical guide to analytical method validation, including measurement uncertainty and accuracy profiles. TrAC Trends Anal Chem. 2007; 26(3):227–238. https://doi.org/10.1016/j.trac.2007.01.009