Wound Healing Potential of Hydrogel Extract of Saccharum spontaneum Leaves Using Incision Wound Healing Model in Rats
Main Article Content
Abstract
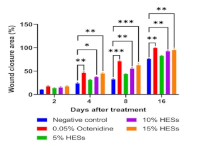
Saccharum spontaneum is a medicinal plant known for treating various diseases, such as wounds. Despite the potential use of this plant, there are no studies confirming its wound-healing activity. This study assessed S. spontaneum extract hydrogel formulation (HESs) and examined its effects on wound healing, antioxidant properties, and antibacterial activity. The disk diffusion method was used to evaluate the antibacterial activity, while DPPH and FRAP were used to measure the antioxidant activity. The rats were divided into 5 groups of 4 rats each to assess wound healing using the incision wound model. Group 1 served as the negative control, while group 2 was given 0.05% octenidine. S. spontaneum extract hydrogel formulation (HESs) was administered to groups 3, 4, and 5 at 5%, 10%, and 15% (%w/w) concentrations, respectively. The results showed that S. spontaneum extract has antioxidant activity with IC50 values of 80.16 (in DPPH) and 82.70 µg/mL (in FRAP), while exhibiting an inhibitory effect on the growth of bacteria in a concentration-dependent manner. The wound healing activity test results indicated that 10% and 15% HESs significantly closed the wound area on days 4, 8, and 16. The S. spontaneum leaf extract exhibited wound healing potential.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.Kalva SN, Augustine R, Al Mamun A, Dalvi YB, Vijay N, Hasan A. Active agents loaded extracellular matrix mimetic electrospun membranes for wound healing applications. J Drug Deliv Sci Technol. 2021; 63:1–16. doi: https://doi.org/10.1016/j.jddst.2021.102500
2.Muharni M, Annisa A, Fitrya F, Anas M. Wound healing activity of Dillenia ochreata leaves ethanol extract in Wistar rats. J Pharm Pharmacogn Res. 2022; 10(5):896–904. doi: https://doi.org/10.56499/jppres22.1444_10.5.896
3.Tessema Z, Molla Y. Evaluation of the wound healing activity of the crude extract of root bark of Brucea antidysentrica, the leaves of Dodonaea angustifolia and Rhamnus prinoides in mice. Heliyon. 2021; 7(1):1–8. doi: https://doi.org/10.1016/j.heliyon.2021.e05901
4.Arun M, Satish S, Anima P. Evaluation of wound healing, antioxidant and antimicrobial efficacy of Jasminum auriculatum Vahl. Avicenna J Phytomed. 2016; 6(3):295–304. doi: https://doi.org/10.22038/ajp.2016.5723
5.Yiblet TG, Tsegaw A, Ahmed N, Dagnew SB, Tadesse TY, Kifle ZD. Evaluation of wound healing activity of 80% methanol root crude extract and solvent fractions of Stephania abyssinica (Dill. & A. Rich.) Walp. (Menispermaceae) in mice. J Exp Pharmacol. 2022; 14:255–273. doi: https://doi.org/10.2147/JEP.S364282
6.Yates CC, Whaley D, Babu R, Zhang J, Krishna P, Beckman E, Pasculle AW, Wells A. The effect of multifunctional polymer-based gels on wound healing in full-thickness bacteria-contaminated mouse skin wound models. Biomaterials. 2007; 28(27):3977–3986. doi: https://doi.org/10.1016/j.biomaterials.2007.05.008
7.Sofrona E, Tziveleka LA, Harizani M, Koroli P, Sfiniadakis I, Roussis V, Rallis M, Ioannou E. In vivo evaluation of the wound healing activity of extracts and bioactive constituents of the marine isopod Ceratothoa oestroides. Mar Drugs. 2020; 18(4):1–15. doi: https://doi.org/10.3390/md18040219
8.Kumari MK, Vittalrao AM, Charitha C, Kumar PSE, Prabhath S. Evaluation of wound healing activity of an ethanolic extract of Anacardium occidentale leaves in Wistar rats. Biomed Pharmacol J. 2020; 13(4):2061–2068. doi: https://dx.doi.org/10.13005/bpj/2086
9.Uberoi A, McCready-Vangi A, Grice EA. The wound microbiota: microbial mechanisms of impaired wound healing and infection. Nat Rev Microbiol. 2024; 22(8):507–521. doi: https://doi.org/10.1038/s41579-024-01035-z
10.Li C, Li C, Ma Z, Chen H, Ruan H, Deng L, Wang J, Cui W. Regulated macrophage immune microenvironment in 3D printed scaffolds for bone tumour postoperative treatment. Bioact Mater. 2022; 19:474–485. doi: https://doi.org/10.1016/j.bioactmat.2022.04.028
11.Sun X, Gao Y, Li Z, He J, Wu Y. Magnetic responsive hydroxyapatite scaffold modulated macrophage polarisation through PPAR/JAK-STAT signalling and enhanced fatty acid metabolism. Biomaterials. 2023; 295:122051. doi: https://doi.org/10.1016/j.biomaterials.2023.122051
12.Deng A, Yang Y, Du S, Yang X, Pang S, Wang X, Yang S. Preparation of a recombinant collagen-peptide (RHC)-conjugated chitosan thermosensitive hydrogel for wound healing. Mater Sci Eng C. 2021; 119:1–13. doi: https://doi.org/10.1016/j.msec.2020.111555
13.Qu J, Zhao X, Liang Y, Xu Y, Ma PX, Guo B. Degradable conductive injectable hydrogels as novel antibacterial, antioxidant wound dressings for wound healing. Chem Eng J. 2019; 362:548–560. doi: https://doi.org/10.1016/j.cej.2019.01.028
14.Wu H, Li F, Wang S, Lu J, Li J, Du Y, Sun X, Chen X, Gao J, Ling D. Ceria nanocrystals decorated mesoporous silica nanoparticle-based ROS-scavenging tissue adhesive for highly efficient regenerative wound healing. Biomaterials. 2018; 151: 66–77. doi: https://doi.org/10.1016/j.biomaterials.2017.10.018
15.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005; 23(1):47–55. doi: https://doi.org/10.1038/nbt1055
16.Halim MBA, Eid HH, Deeb KSE, Metwally GF, Masoud MA, Ahmed-Farid OA, Messiry HME. The study of wound healing activity of Thespesia populnea L. bark, an approach for accelerating healing through nanoparticles and isolation of main active constituents. BMC Complement Med Ther. 2024; 24(1):1–16. doi: https://doi.org/10.1186/s12906-024-04343-2
17.Thakur R, Jain N, Pathak R, Sandhu SS. Practices in wound healing studies of plants. Evid Based Complement Alternat Med. 2011; 2011:1–17. doi: https://doi.org/10.1155/2011/438056
18.Cedillo-Cortezano M, Martinez-Cuevas LR, López JAM, Barrera López IL, Escutia-Perez S, Petricevich VL. Use of medicinal plants in the process of wound healing: A literature review. Pharmaceuticals. 2024; 17(3):1–27. doi: https://doi.org/10.3390/ph17030303
19.Hassan A, Malik K, Naqvi SAM, Sadia H, Khan K. A comprehensive review of Saccharum spontaneum, its traditional uses, phytochemistry and pharmacology. Ethnobot Res Appl. 2024; 29(5):1–13. doi: http://dx.doi.org/10.32859/era.29.5.1-13
20.Okokon JE, Udobang JA, Davies K, Edem UA, Bassey AI. Analgesic activity of ethanol leaf extract of Saccharum officinarum. Trop J Nat Prod Res. 2021; 5(6):1142–1145. doi: https://doi.org/10.26538/tjnpr/v5i6.27
21.Zagórska-Dziok M, Sobczak M. Hydrogel-based active substance release systems for cosmetology and dermatology application: A review. Pharmaceutics. 2020; 12(5):1–43. doi: https://doi.org/10.3390/pharmaceutics12050396
22.Alberts A, Moldoveanu ET, Niculescu AG, Grumezescu AM. Hydrogels for wound dressings: Applications in burn treatment and chronic wound care. J Compos Sci. 2025; 9(3):1–37. doi: https://doi.org/10.3390/jcs9030133
23.Alkandahri MY, Maulana YE, Subarnas A, Kwarteng A, Berbudi A. Antimalarial activity of extract and fractions of Cayratia trifolia (L.) Domin. Int J Pharm Res. 2020; 12(Suppl 1):1435–1441. doi: https://doi.org/10.31838/ijpr/2020.SP1.218
24.Gunarti NS, Alkandahri MY, Wahyuningsih ES, Agustina P, Mursal ILP, Hidayah H, Nurviana V. Evaluation of antipyretic and antioxidant activities of ten indigenous medicinal plants of Tirtajaya, Karawang Regency, West Java, Indonesia. Indian J Pharm Educ Res. 2025; 59(1):252–263. doi: https://doi.org/10.5530/ijper.20256381
25.Alkandahri MY, Kusumiyati K, Renggana H, Arfania M, Frianto D, Wahyuningsih ES, Maulana YE. Antihyperlipidemic activity of extract and fractions of Castanopsis costata leaves on rats fed with high cholesterol diet. RASĀYAN J Chem. 2022; 15(4):2350–2358. doi: http://doi.org/10.31788/RJC.2022.1547015
26.Sytar O, Hemmerich I, Zivcak M, Rauh C, Brestic M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J Biol Sci. 2018; 25(4):631–641. doi: https://doi.org/10.1016/j.sjbs.2016.01.036
27.Alkandahri MY, Sadino A, Abriyani E, Hermanto F, Oktoba Z, Sayoeti MFW, Sangging PRA, Wardani D, Hasan N, Sari SW, Safitri NA, Ikhtianingsih W, Safitri S. Evaluation of hepatoprotective and nephroprotective activities of Castanopsis costata extract in rats. Biomed Rep. 2025; 22(2):1–11. doi: https://doi.org/10.3892/br.2024.1902
28.Yuniarsih N, Hidayah H, Gunarti NS, Kusumawati AH, Farhamzah F, Sadino A, Alkandahri MY. Evaluation of wound-healing activity of hydrogel extract of Sansevieria trifasciata leaves (Asparagaceae). Adv Pharmacol Pharm Sci. 2023; 2023:1–10. doi: https://doi.org/10.1155/2023/7680518
29.Forestryana D, Hayati A, Putri AN. Formulation and evaluation of natural gel containing ethanolic extract of Pandanus amaryllifolius R. using various gelling agents. Borneo J Pharm. 2022; 5(4):345–356. doi: https://doi.org/10.33084/bjop.v5i4.1411
30.Nurman S, Yulia R, Irmayanti, Noor E, Sunarti TC. The optimisation of gel preparations using the active compounds of arabica coffee ground nanoparticles. Sci Pharm. 2019; 87(4):1–16. doi: https://doi.org/10.3390/scipharm87040032
31.Bonacucina G, Cespi M, Palmieri GF. Characterisation and stability of emulsion gels based on acrylamide/sodium acryloyldimethyl taurate copolymer. AAPS PharmSciTech. 2009; 10(2):368–375. doi: https://doi.org/10.1208/s12249-009-9218-1
32.Edityaningrum CA, Kintoko, Zulien F, Widiyastuti L. Optimisation of water fraction gel formula of binahong leaf (Anredera cordifolia (Ten.) Steen) with gelling agent of sodium alginate and carboxymethyl chitosan combination. Trad Med J. 2018; 23(3):97–105. doi: https://doi.org/10.22146/mot.36604
33.Farhamzah, Kusumawati AH, Alkandahri MY, Hidayah H, Sujana D, Gunarti NS, Yuniarsih N, Apriana SD, Agustina LS. Sun protection factor activity of black glutinous rice emulgel extract (Oryza sativa var glutinosa). Indian J Pharm Educ Res. 2022; 56(1):302–310. doi: https://doi.org/10.5530/ijper.56.1.36
34.Kusumawati AH, Farhamzah F, Alkandahri MY, Sadino A, Agustina LS, Apriana SD. Antioxidant activity and sun protection factor of Black Glutinous Rice (Oryza sativa var. glutinosa). Trop J Nat Prod Res. 2021; 5(11):1958–1961. doi: https://doi.org/10.26538/tjnpr/v5i11.11
35.Tamuly C, Hazarika M, Bora J, Gajurel PR. Antioxidant activities and phenolic content of Piper wallichii (Miq.) Hand.-Mazz. Int J Food Prop. 2014; 17(2):309–320. doi: https://doi.org/10.1080/10942912.2011.631250
36.Pertiwi D, Hartati R, Julianti E, Fidrianny I. Antibacterial and antioxidant activities in various parts of Artocarpus lacucha Buch. Ham. Ethanolic extract. Biomed Rep. 2024; 20(4):1–11. doi: https://doi.org/10.3892/br.2024.1755
37.Moglad EH, Hamad AM, Fatima F, Seshadri VD, Naz M. Antimicrobial and wound healing activities of certain Sudanese medicinal plants. Saudi J Biol Sci. 2020; 27(7):1766–1772. doi: https://doi.org/10.1016/j.sjbs.2020.05.017
38.Lambebo MK, Kifle ZD, Gurji TB, Yesuf JS. Evaluation of wound healing activity of methanolic crude extract and solvent fractions of the leaves of Vernonia auriculifera Hiern (Asteraceae) in mice. J Exp Pharmacol. 2021; 13:677–692. doi: https://doi.org/10.2147/JEP.S308303
39.Belachew TF, Asrade S, Geta M, Fentahun E. In vivo evaluation of wound healing and anti-inflammatory activity of 80% methanol crude flower extract of Hagenia abyssinica (Bruce) J.F. Gmel in mice. Evid Based Complement Alternat Med. 2020; 2020:1–12. doi: https://doi.org/10.1155/2020/9645792
40.Tsimogiannis DI, Oreopoulou V. The contribution of flavonoid Cring on the DPPH free radical scavenging efficiency. A kinetic approach for the 3’,4’hydroxy substituted members. Innov Food Sci Emerg Technol. 2006; 7(1-2):140–146. doi: https://doi.org/10.1016/j.ifset.2005.09.001
41.Platzer M, Kiese S, Tybussek T, Herfellner T, Schneider F, Schweiggert-Weisz U, Eisner P. Radical scavenging mechanisms of phenolic compounds: A quantitative structure-property relationship (QSPR) study. Front Nutr. 2022; 9:1–12. doi: https://doi.org/10.3389/fnut.2022.882458
42.Gulcin I, Alwasel SH. DPPH radical scavenging assay. Processes. 2023; 11(8):1–20. doi: https://doi.org/10.3390/pr11082248
43.Alkandahri MY, Arfania M, Abriyani E, Ridwanuloh D, Farhamzah F, Fikayuniar L, Hasyim DM, Nurul, Wardani D. Evaluation of antioxidant and antipyretic effects of ethanolic extract of cepcepan leaves (Castanopsis costata (Blume) A.D.C.). J Adv Pharm Educ Res. 2022; 12(3):107–112. doi: https://doi.org/10.51847/twcOIyzqTM
44.Spiegel M, Kapusta K, Kołodziejczyk W, Saloni J, Żbikowska B, Hill GA, Sroka Z. Antioxidant activity of selected phenolic acids-ferric reducing antioxidant power assay and QSAR analysis of the structural features. Molecules. 2020; 25(13):1–15. doi: https://doi.org/10.3390/molecules25133088
45.Gonzalez AC, Costa TF, Andrade ZA, Medrado AR. Wound healing - A literature review. An Bras Dermatol. 2016; 91(5):614–620. doi: https://doi.org/10.1590/abd1806-4841.20164741
46.Dehkordi AN, Babaheydari FM, Chehelgerdi M, Dehkordi SR. Skin tissue engineering: wound healing based on stem-cell-based therapeutic strategies. Stem Cell Res Ther. 2019; 10:1–20. doi: https://doi.org/10.1186/s13287-019-1212-2
47.Subramanian S, Duraipandian C, Alsayari A, Ramachawolran G, Wong LS, Sekar M, Gan SH, Subramaniyan V, Seethalakshmi S, Jeyabalan S, Dhanasekaran S, Chinni SV, Rani NNIM, Wahab S. Wound healing properties of a new formulated flavonoid-rich fraction from Dodonaea viscosa Jacq. Leaves extract. Front Pharmacol. 2023; 14:1–16. doi: https://doi.org/10.3389/fphar.2023.1096905
48.Tottoli EM, Dorati R, Genta I, Chiesa E, Pisani S, Conti B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics. 2020; 12(8):1–30. doi: https://doi.org/10.3390/pharmaceutics12080735
49.Choudhary V, Choudhary M, Bollag WB. Exploring skin wound healing models and the impact of natural lipids on the healing process. Int J Mol Sci. 2024; 25(7):1–29. doi: https://doi.org/10.3390/ijms25073790
50.Demilew W, Adinew GM, Asrade S. Evaluation of the wound healing activity of the crude extract of leaves of Acanthus polystachyus Delile (Acanthaceae). Evid Based Complement Alternat Med. 2018; 2018:1–9. doi: https://doi.org/10.1155/2018/2047896
51.Kanlayavattanakul M, Khongkow M, Lourith N. Wound healing and photoprotection properties of Acanthus ebracteatus Vahl. extracts standardised in verbascoside. Sci Rep. 2024; 14(1):1–9. doi: https://doi.org/10.1038/s41598-024-52511-8
52.Taufik AY, Yasin HM, Ahmad N, Arai M, Ja’afar F. An investigation into the phytochemical content and antioxidant, antidiabetic, and wound-healing activities of Curculigo latifolia found in Brunei Darussalam. Sci World J. 2024; 2024:1–22. doi: https://doi.org/10.1155/2024/5656744
53.Alkandahri MY, Sujana D, Hasyim DM, Shafirany MZ, Sulastri L, Arfania M, Frianto D, Farhamzah, Kusumawati AH, Yuniarsih N. Antidiabetic activity of extract and fractions of Castanopsis costata leaves on alloxan-induced diabetic mice. Pharmacogn J. 2021; 13(6 Suppl):1589–1593. doi: https://doi.org/10.5530/pj.2021.13.204
54.Tekleyes B, Huluka SA, Wondu K, Wondmkun YT. Wound healing activity of 80% methanol leaf extract of Zehneria scabra (L.f) Sond (Cucurbitaceae) in mice. J Exp Pharmacol. 2021; 13:537–544. doi: https://doi.org/10.2147/JEP.S303808
55.Carvalho MTB, Araújo-Filho HG, Barreto AS, Quintans-Júnior LJ, Quintans JSS, Barreto RSS. Wound healing properties of flavonoids: A systematic review highlighting the mechanisms of action. Phytomedicine. 2021; 90:153636. doi: https://doi.org/10.1016/j.phymed.2021.153636
56.Alkandahri MY, Pamungkas BT, Oktoba Z, Shafirany MZ, Sulastri L, Arfania M, Anggraeny EN, Pratiwi A, Astuti FD, Indriyani, Dewi SY, Hamidah SZ. Hepatoprotective effect of kaempferol: A review of the dietary sources, bioavailability, mechanisms of action, and safety. Adv Pharmacol Pharm Sci. 2023; 2023:1–16. doi: https://doi.org/10.1155/2023/1387665
57.Alkandahri MY, Sadino A, Pamungkas BT, Oktoba Z, Arfania M, Yuniarsih N, Wahyuningsih ES, Dewi Y, Winarti SA, Dinita ST. Potential nephroprotective effect of kaempferol: Biosynthesis, mechanisms of action, and clinical prospects. Adv Pharmacol Pharm Sci. 2024; 2024:1–17. doi: https://doi.org/10.1155/2024/8907717
58.Alkandahri MY, Berbudi A, Subarnas A. Active compounds and antimalaria properties of some medicinal plants in Indonesia – A review. Sys Rev Pharm. 2018; 9(1):64–69. doi: https://doi.org/10.5530/srp.2018.1.13
59.Kumari P, Singh V, Kant V, Ahuja M. Current status of 1,4-Naphthoquinones and their derivatives for wound healing. Eur J Med Chem Rep. 2024; 12:1–23. doi: https://doi.org/10.1016/j.ejmcr.2024.100194
60.Liu E, Gao H, Zhao Y, Pang Y, Yao Y, Yang Z, Zhang X, Wang Y, Yang S, Ma X, Zeng J, Guo J. The potential application of natural products in cutaneous wound healing: A review of preclinical evidence. Front Pharmacol. 2022; 13:1–18. doi: https://doi.org/10.3389/fphar.2022.900439
61.Khan MI, Karima G, Khan MZ, Shin JH, Kim JD. Therapeutic effects of saponins for the prevention and treatment of cancer by ameliorating inflammation and angiogenesis and inducing antioxidant and apoptotic effects in human cells. Int J Mol Sci. 2022; 23(18):1–15. doi: https://doi.org/10.3390/ijms231810665
62.Men SY, Huo QL, Shi L, Yan Y, Yang CC, Yu W, Liu BQ. Panax notoginseng saponins promote cutaneous wound healing and suppress scar formation in mice. J Cosmet Dermatol. 2020; 19(2):529–534. doi: https://doi.org/10.1111/jocd.13042
63.Budiawan A, Purwanto A, Puradewa L, Cahyani ED, Purwaningsih CE. Wound healing activity and flavonoid contents of purslane (Portulaca grandiflora) of various varieties. RSC Adv. 2023; 13(15):9871–9877. doi: https://doi.org/10.1039/d3ra00868a
64.Shi XQ, Chen G, Tan JQ, Li Z, Chen SM, He JH, Zhang L, Xu HX. Total alkaloid fraction of Leonurus japonicus Houtt. Promotes angiogenesis and wound healing through SRC/MEK/ERK signalling pathway. J Ethnopharmacol. 2022; 295:115396. doi: https://doi.org/10.1016/j.jep.2022.115396
65.Vyas KS, Vasconez HC. Wound healing: biologics, skin substitutes, biomembranes and scaffolds. Healthcare. 2014; 2(3):356–400. doi: https://doi.org/10.3390/healthcare2030356
66.Scherer MMC, Marques FM, Figueira MM, Peisino MCO, Schmitt EFP, Kondratyuk TP, Endringer DC, Scherer R, Fronza M. Wound healing activity of terpinolene and α-phellandrene by attenuating inflammation and oxidative stress in vitro. J Tissue Viability. 2019; 28(2):94–99. doi: https://doi.org/10.1016/j.jtv.2019.02.003
67.Wang G, Yang F, Zhou W, Xiao N, Luo M, Tang Z. The initiation of oxidative stress and therapeutic strategies in wound healing. Biomed Pharmacother. 2023; 157:1–12. doi: https://doi.org/10.1016/j.biopha.2022.114004
68.Puca V, Marulli RZ, Grande R, Vitale I, Niro A, Molinaro G, Prezioso S, Muraro R, Giovanni, PD. Microbial species isolated from infected wounds and antimicrobial resistance analysis: Data emerging from a three-year retrospective study. Antibiotics. 2021; 10(10):1–14. doi: https://doi.org/10.3390/antibiotics10101162
69.Comino-Sanz IM, López-Franco MD, Castro B, Pancorbo-Hidalgo PL. The role of antioxidants on wound healing: A review of the current evidence. J Clin Med. 2021; 10(16):1–22. doi: https://doi.org/10.3390/jcm10163558
70.Deng X, Gould M, Ali MA. A review of current advancements for wound healing: Biomaterial applications and medical devices. J Biomed Mater Res B Appl Biomater. 2022; 110(11):2542–2573. doi: https://doi.org/10.1002/jbm.b.35086
71.Hassanpour SH, Doroudi A. Review of the antioxidant potential of flavonoids as a subgroup of polyphenols and partial substitute for synthetic antioxidants. Avicenna J Phytomed. 2023; 13(4):354–376. doi: https://doi.org/10.22038/AJP.2023.21774
72.Chen Q, Wang J, Gao Y, Wang Z, Wang D, Gao X, Yan P. Fermentation, identification, and antioxidant activity of saponins produced by a wild ginseng endophytic fungus Umbelopsis dimorpha Strain NSJG. Fermentation. 2024; 10(1):1–13; https://doi.org/10.3390/fermentation10010009
73.Xie Y, Yang W, Tang F, Chen X, Ren L. Antibacterial activities of flavonoids: Structure-activity relationship and mechanism. Curr Med Chem. 2015; 22(1):132–149. doi: https://doi.org/10.2174/0929867321666140916113443
74.Li J, Monje-Galvan V. In vitro and in silico studies of antimicrobial saponins: A review. Processes. 2023; 11(10):1–17. doi: https://doi.org/10.3390/pr11102856
75.Cores Á, Carmona-Zafra N, Clerigué J, Villacampa M, Menéndez JC. Quinones as neuroprotective agents. Antioxidants. 2023; 12(7):1–37. doi: https://doi.org/10.3390/antiox12071464
76.Sirin S, Dolanbay SN, Aslim B. Role of plant-derived alkaloids as antioxidant agents for neurodegenerative diseases. Health Sci Rev. 2023; 6:1–11. doi: https://doi.org/10.1016/j.hsr.2022.100071
77.Kokilananthan S, Bulugahapitiya VP, Manawadu H, Gangabadage CS. Sesquiterpenes and monoterpenes from different varieties of guava leaf essential oils and their antioxidant potential. Heliyon. 2022; 8(12):1–9. doi: https://doi.org/10.1016/j.heliyon.2022.e12104
78.Junior MAD, Edzang RWN, Catto AL, Raimundo JM. Quinones as an efficient molecular scaffold in the antibacterial/antifungal or antitumoral arsenal. Int J Mol Sci. 2022; 23(22):1–16. doi: https://doi.org/10.3390/ijms232214108
79.Yan Y, Li X, Zhang C, Lv L, Gao B, Li M. Research progress on antibacterial activities and mechanisms of natural alkaloids: A review. Antibiotics. 2021; 10(3):1–30. doi: https://doi.org/10.3390/antibiotics10030318
80.Huang W, Wang Y, Tian W, Cui X, Tu P, Li J, Shi S, Liu X. Biosynthesis investigations of terpenoid, alkaloid, and flavonoid antimicrobial agents derived from medicinal plants. Antibiotics. 2022; 11(10):1–32. doi: https://doi.org/10.3390/antibiotics11101380