Therapeutic Efficacy of β-Hydroxybutyrate Supplementation on Weight Loss and Glycemic Control in Fasted Obese and Diabetic Mice
Main Article Content
Abstract
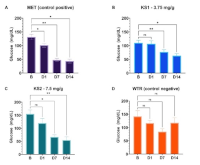
The global prevalence of obesity and diabetes necessitates novel therapeutic interventions. Ketone bodies, particularly beta-hydroxybutyrate (BHB), have emerged as promising agents for metabolic management. This study assessed how effective the efficacy of oral beta-hydroxybutyrate (BHB) supplementation on weight loss and glycemic control in a streptozotocin (STZ; 50 mg/kg, intraperitoneal) mouse model of obesity and diabetes induced by a high-fat diet (HFD, which consists of 45% beef tallow, 35% egg yolk, and 20% commercial food pellets) for 21 days under a 16-hour daily fasting protocol. Male Swiss Webster mice were divided into four groups and orally administered: metformin (0.1 mg/g) (positive control), low-dose BHB (3.75 mg/g), high-dose BHB (7.5 mg/g), and water (negative control). All treatments were administered once daily for 14 days. At the end of the treatment period, parameters evaluated included final body weight, fasting blood glucose (FBG) levels in the tail vein on days 0, 1, 7, and 14, and urinary ketone levels via urine sampling. Results demonstrated that high-dose BHB administration induced a significantly reduced body weight (p < 0.05) and fasting blood glucose levels (p < 0.01), with efficacy comparable to that of metformin. The low-dose BHB group showed a statistically significant improvement in fasting blood glucose (FBG) (p < 0.05) but did not significantly reduce body weight. Critically, urinary ketones remained undetectable, indicating no induction of ketoacidosis. These results suggest that oral BHB supplementation, particularly at higher doses, is a safe and effective strategy for improving metabolic outcomes during fasting in obese and diabetic models, positioning it as a viable adjunct therapeutic option.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.Gabriel AO, Ogbadu DE. Anti-obesity Effect of Ocimum gratissimum leaf Powder Supplementation on High Fat Diet-induced Obesity in Male Wistar Rats. Trop J Nat Prod Res. 2024;8(6):7397–73402. Doi: 10.26538/tjnpr/v8i6.9.
2.Binh NTM., Tuan NTH, Ngan NH, Huy THA, Tien DP, Hue LT, Giang DH, Toan TD, Phuong PT. The Effects of Camellia hakodae Ninh Dried Leaf Extract on Obesity in Rats Induced by a High-Fat Diet. Trop J Nat Prod Res. 2025;7(9). Doi: 10.26538/tjnpr/v9i7.40.
3.International Diabetes Federation. IDF Global Clinical Practice Recommendations for Managing Type 2 Diabetes 2025. Brussels, Belgium: International Diabetes Federation, 2025. Available from: https://idf.org/media/uploads/2025/04/IDF_Rec_2025.pdf.
4.Arifani S, Setiyaningrum Z. Risk Factors Associated with Obesity in Adults in Banten Province in 2018. J Heal. 2021;14(2):160–168. Doi: 10.23917/jk.v14i2.13738.
5.Setiawan MD, Susilawati. The effect of diabetes self-management education programs on patients with type 2 diabetes mellitus in Indonesia (a: systematic review). Nautical Ind Multidisciplinary Sci J. 2022;1(3):132–138.
6.Vasim I, Majeed CN, DeBoer MD. Intermittent Fasting and Metabolic Health. Nutrients. 2022;14(3):1–15. Doi: 10.3390/ nu14030631.
7.Crosby L, Davis B, Joshi S, Jardine M, Paul J, Neola M, Barnard ND. Ketogenic Diets and Chronic Disease: Weighing the Benefits Against the Risks. Front Nutr. 2021;8:1–11. Doi: 10.3389/fnut.2021.702802.
8.Falkenhain K, Daraei A, Forbes SC, Little JP. Effects of Exogenous Ketone Supplementation on Blood Glucose: A Systematic Review and Meta-analysis. Adv Nutr. 2022;13(5):1697–1714. Doi: 10.1093/advances/nmac036
9.Soto‐Mota A, Norwitz NG, Evans RD, Clarke K. Exogenous d‐β‐hydroxybutyrate lowers blood glucose in part by decreasing the availability of L‐alanine for gluconeogenesis. Endocrinol Diabetes Metab. 2022;5(1):1–8. Doi: 10.1002/edm2.300.
10.Pimentel-Suarez LI, Soto-Mota A. Evaluation of the safety and tolerability of exogenous ketosis induced by orally administered free beta-hydroxybutyrate in healthy adult subjects. BMJ Nutr Prev Health. 2023;6(2):122–126. Doi: 10.1136/bmjnph-2023-000672.
11.Cuenoud B, Hartweg M, Godin JP, Croteau E, Maltais M, Castellano CA, Carpentier AC, Cunnane SC. Metabolism of Exogenous D-Beta-Hydroxybutyrate, an Energy Substrate Avidly Consumed by the Heart and Kidney. Front Nutr. 2020;7(13):1–19. Doi: 10.3389/fnut.2020.00013.
12.Gotera W, Nugraha IBA, Sugitha KSL. Benefits of the Ketogenic Diet in Obesity Management. Mir Med World. 2023;50(8):451–458.
13.Crabtree CD, Kackley ML, Buga A, Fell B, LaFountain RA, Hyde PN, Sapper PN, Kraemer WJ, Scandling D, Simonetti OP, Volek JS. Comparison of Ketogenic Diets with and without Ketone Salts versus a Low-Fat Diet: Liver Fat Responses in Overweight Adults. Nutrients. 2021;13(966):1–14. Doi: 10.3390/nu13030966.
14.Kesl SL, Angela M. Poff, Ward NP, Fiorelli TN, Ari C, Van Putten AJ, Sherwood JW, Arnold P, D'Agustino DP. Effects of exogenous ketone supplementation on blood ketone, glucose, triglyceride, and lipoprotein levels in Sprague–Dawley rats. Nutr Metab (Lond). 2016 Dec;13(9):1–15. Doi: 10.1186/s12986-016-0069-y.
15.Buga A, Kackley ML, Crabtree CD, Sapper TN, Mccabe L, Fell B, LaFountain RA, Hyde PN, Martini ER, Bowman J, Pan Y, Scandling D, Brownlow ML, O'Connor A, Simonetti OP, Kraemer WJ, Volek JS. The Effects of a 6-Week Controlled, Hypocaloric Ketogenic Diet, With and Without Exogenous Ketone Salts, on Body Composition Responses. Front Nutr. 2021;8:618520. Doi: 10.3389/fnut.2021.618520.
16.Magfirah, Utami IK, Alaydrus S. Effect of Seaweed Ethanol Extract on Cholesterol Levels and Obesity in White Rats. J Jamu Indo. 2020;5(3):98–105. Doi: 10.29244/jji.v5i3.175
17.Lim SM, Goh YM, Mohtarrudin N, Loh SP. Germinated brown rice ameliorates obesity in high-fat diet induced obese rats. BMC Complement Altern Med. 2016;16(140):1–11. DOI 10.1186/s12906-016-1116-y.
18.Sasongko H, Rohman A, Nurrochmad A, Nugroho A. Biochemical And Triglyceride-Glucose Index (Tyg) Profile In High Doses Streptozotocin-Nicotinamide Produce Diabetes Mellitus In Rats Model. Trop J Nat Prod Res. 2024;8(6). Doi: 10.26538/tjnpr/v8i6.25.
19.Djuric DM, Gtaric N, Todorovic D, Sankovic S, Dragičević-Cvjetković D, Dragičević-Cvjetković MP, Škrbić R, Vučković S. The Effects of Subchronic Intake of Magnesium Hydro- carbonate-Rich Mineral Water on Cardiometabolic Markers and Electrolytes in Rats With Streptozotocin-Induced Diabetes. Scrip Med. 2022; 53(3), 197–204. Doi: 10.5937/scriptamed53-40112.
20.Jaishree V, Narsimha S. Swertiamarin and quercetin combination ameliorates hyperglycemia, hyperlipidemia and oxidative stress in streptozotocin-induced type 2 diabetes mellitus in wistar rats. Biomed Pharmacother. 2020 Oct;130:110561. Doi: 10.1016/j.biopha.2020.110561.
21.Nufus I, Qomariyah N, Purnama ER. Antidiabetic Activity of Manila Sapodilla Leaf Extract on Blood Sugar Levels and Healing of Diabetic Mouse Ulcers. LenteraBio. 2021;10(3):319–328.
22.Dias MDME, Reis SAD, Conceição LLD, Sediyama CMNDO, Pereira SS, De Oliveira LL, Peluzio MDCG, Martinez JA, Milagro FI. Diet-induced obesity in animal models: points to consider and influence on metabolic markers. Diabetol Metab Syndr. 2021;13(32):1–14. Doi: 10.1186/s13098-021-00647-2.
23.Rosnah R, Taslim NA, Aman AM, Idris I, As’ad S, Buchari A, Bahar , Aminudin, Wahyudin E, Nugraha GI. The Formulation and Evaluation of High-Fat Pellet on Lipid Profiles and Body Mass Index of Male Wistar Rats. Int J Pharm Sci. 2022;31(1):285–292. Doi: 10.31351/vol31iss1pp285-292.
24.Akinlade OM, Owoyele BV, Soladoye AO. Streptozotocin-induced type 1 and 2 diabetes in rodents: a model for studying diabetic cardiac autonomic neuropathy. Afr H Sci. 2021;21(2):719–727. Doi: 10.4314/ahs.v21i2.30.
25.Liu X, Du P, Xu J, Wang W, Zhang C. Therapeutic Effects of Intermittent Fasting Combined with SLBZS and Prebiotics on STZ-HFD-Induced Type 2 Diabetic Mice. Diabetes Metab Syndr Obes (DMSO). 2024;17:4013–4030. Doi: 10. 2147/DMSO. S474196.
26.Holcomb LE, O’Neill CC, DeWitt EA, Kolwicz SC. The Effects of Fasting or Ketogenic Diet on Endurance Exercise Performance and Metabolism in Female Mice. Metabolites. 2021;11(397):1–14. Doi: 10.3390/ metabo11060397.
27.Stubbs BJ, Cox PJ, Evans RD, Santer P, Miller JJ, Faull OK, Magor-Elliott S, Hiyama S, Stirling M, Clarke K. On the Metabolism of Exogenous Ketones in Humans. Front Physiol. 2017;8:1–13. Doi: 10.3389/fphys.2017.00848.
28.Salomo H. The Potential Use of Metformin as a Dietary Supplement for Obesity. Ind Med Stud J. 2020;8(1):38-43. Doi: 10.53366/jimki.v8i1.35.
29.Dutta S, Shah RB, Singhal S, Dutta SB, Bansal S, Sinha S, Haque M. Metformin: A Review of Potential Mechanism and Therapeutic Utility Beyond Diabetes. DDDT. 2023; 17:1907–1932. Doi: 10. 2147/DDD T. S409373.
30.Yendapally R, Sikazwe D, Kim SS, Ramsinghani S, Fraser‐Spears R, Witte AP, La-Viola B. A review of phenformin, metformin, and imeglimin. Drug Dev Res. 2020;81(4):390–401. Doi: 10.1002/ddr.21636.
31.Kim EK, Lee SH, Jhun JY, Byun JK, Jeong JH, Lee SY, Kim JK, Choi JY, Cho M. Metformin Prevents Fatty Liver and Improves Balance of White/Brown Adipose in an Obesity Mouse Model by Inducing FGF21. Mediators Inflam. 2016;2016:1–13. Doi: 10.1155/2016/5813030.
32.Cavaleri F, Bashar E. Potential Synergies of β-Hydroxybutyrate and Butyrate on the Modulation of Metabolism, Inflammation, Cognition, and General Health. J nutr metab. 2018;7195760:1–13. Doi: 10.1155/2018/7195760.
33.Zhao MF, Zhang XG, Tang YP, Zhu YX, Nie HY, Bu DD, Fang L, Li CJ. Ketone bodies promote epididymal white adipose expansion to alleviate liver steatosis in response to a ketogenic diet. J Biol Chem. 2024;300(2):105617. 10.1016/j.jbc.2023.105617.
34.Martins C, Nymo S, Truby H, Rehfeld JF, Hunter GR, Gower BA. Association Between Ketosis and Changes in Appetite Markers with Weight Loss Following a Very Low‐Energy Diet. Obesity. 2020;28(12):2331–2338. Doi: 10.1002/oby.23011.
35.Khan ES, Karanjkar P, Kishore VNR. Novel quantitative assay for estimation of ketone bodies in diabetic urine. Int J of Sci Eng Res. 2016; 7(9), 701–705.