Bidirectional Conversion Models to Harmonize Total Phenolic and Flavonoid Content Analysis in Medicinal Plant Extracts
Main Article Content
Abstract
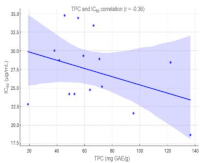
Accurate quantification of total phenolic content (TPC) and total flavonoid content (TFC) is essential for evaluating the quality and biological potential of medicinal plant extracts. However, variations in reference standards and assay conditions often lead to inconsistent data across laboratories. Therefore, this study aimed to develop bidirectional conversion models to harmonize TPC and TFC values obtained using different calibration standards and compare with previous reports. A total of 15 Indonesian medicinal plant extracts were analyzed using UV–Visible spectrophotometry, with seven standards for TPC (gallic acid, catechin, quercetin, rutin, apigenin, kaempferol, and myricetin) and five TFC (quercetin, rutin, apigenin, kaempferol, and myricetin). Linear regression models (R2 > 0.995) were established to interconvert values among these standards. The results showed that there was a strong positive correlation (r = 0.89) between TPC and TFC, while a weak negative relationship was observed with antioxidant activity (IC50). This indicated that only total content did not reliably predict antioxidant capacity. The bidirectional conversion models enabled reliable transformation of data obtained using different reference compounds, facilitating data harmonization across laboratories and studies. This method provided a practical tool for standardizing spectrophotometric assays in natural products and supported the development of consistent phytochemical databases as well as quality control systems for herbal materials.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.Blainski, A.; Lopes, G.C.; De Mello, J.C.P. Application and Analysis of the Folin Ciocalteu Method for the Determination of the Total Phenolic Content from Limonium Brasiliense L. Molecules 2013; 18(6):6852-6865. https://doi.org/10.3390/molecules18066852
2.Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005; 53(10):4290-4302. https://doi.org/10.1021/jf0502698
3.Frahm, E.; Wright, J. Evaluation of Inter-Laboratory Comparison Results: Representative Examples. Measurement 2023; 223:113723. https://doi.org/10.1016/j.measurement.2023.113723
4.Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med 1996; 20(7):933-956. https://doi.org/10.1016/0891-5849(95)02227-9
5.George, J.; Edwards, D.; Pun, S.; Williams, D. Evaluation of Antioxidant Capacity (ABTS and CUPRAC) and Total Phenolic Content (Folin-Ciocalteu) Assays of Selected Fruit, Vegetables, and Spices. Int J Food Sci 2022; 2022(1):2581470. https://doi.org/10.1155/2022/2581470
6.Janiak, M.A.; Gryn-Rynko, A.; Sulewska, K.; Amarowicz, R.; Penkacik, K.; Graczyk, R.; Olszewska-Słonina, D.; Majewski, M.S. Phenolic profiles and antioxidant activity of Morus alba L. infusions prepared from commercially available products and naturally collected leaves. Sci Rep 2025; 15(1):13030. https://doi.org/10.1038/s41598-025-97223-9
7.Tang, S.; Wang, B.; Liu, X.; Xi, W.; Yue, Y.; Tan, X.; Bai, J.; Huang, L. Structural insights and biological activities of flavonoids: Implications for novel applications. Food Front 2025; 6(1):218-247. https://doi.org/10.1002/fft2.494
8.Belew, A.A.; Hanan, G.G.M.W.; Meshesha, D.S.; Akele, M.L. Evaluation of total phenolic, flavonoid contents, antioxidant and antibacterial activity of leaf extracts from Rhus vulgaris. Discov Plant 2025; 2(1):141. https://doi.org/10.1007/s44372-025-00222-3
9.Ran, Y.; Li, F.; Xu, Z.; Zeng, K.; Ming, J. Recent advances in dietary polyphenols (DPs): antioxidant activities, nutrient interactions, delivery systems, and potential applications. Food Funct 2024; 15(20):10213-10232. https://doi.org/10.1039/d4fo02111h
10.Kurek-Górecka, A.; Keskin, Ş.; Bobis, O.; Felitti, R.; Górecki, M.; Otręba, M.; Stojko, J.; Olczyk, P.; Kolayli, S.; Rzepecka-Stojko, A. Comparison of the Antioxidant Activity of Propolis Samples from Different Geographical Regions. Plants 2022; 11(9):1203. https://doi.org/10.3390/plants11091203
11.Lee, J.E.; Jayakody, J.T.M.; Kim, J.I.; Jeong, J.W.; Choi, K.M.; Kim, T.S.; Seo, C.; Azimi, I.; Hyun, J.M.; Ryu, B.M. The Influence of Solvent Choice on the Extraction of Bioactive Compounds from Asteraceae: A Comparative Review. Foods 2024; 13(19). https://doi.org/10.3390/foods13193151
12.Dubale, S.; Kebebe, D.; Zeynudin, A.; Abdissa, N.; Suleman, S. Phytochemical Screening and Antimicrobial Activity Evaluation of Selected Medicinal Plants in Ethiopia. J Exp Pharmacol 2023; 15:51-62. https://doi.org/10.2147/JEP.S379805
13.Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: improvement of its specificity for total phenolic content determination. Anal. Methods 2013; 5(21):5990-5999. https://doi.org/10.1039/C3AY41125G
14.Shraim, A.M.; Ahmed, T.A.; Rahman, M.M.; Hijji, Y.M. Determination of total flavonoid content by aluminum chloride assay: A critical evaluation. LWT 2021; 150:111932. https://doi.org/10.1016/j.lwt.2021.111932
15.Evtyugin, D.D.; Evtuguin, D.V. A comprehensive DPPH study on the antioxidant activity of ellagic acid from Eucalyptus sulphite pulping liquor for food applications. Food Bioprod. Process. 2025; 154:350-358. https://doi.org/10.1016/j.fbp.2025.10.005
16.Mehmood, A.; Javid, S.; Khan, M.F.; Ahmad, K.S.; Mustafa, A. In vitro total phenolics, total flavonoids, antioxidant and antibacterial activities of selected medicinal plants using different solvent systems. BMC Chemistry 2022; 16(1):64. https://doi.org/10.1186/s13065-022-00858-2
17.Arab, Z.A.; Nouri, M.; Asadi, G. Optimization of microwave-assisted extraction for horseradish (Armoracia rusticana) roots: phytochemical components and bioavailability. Biomass COnv. Bioref. 2025. https://doi/org/10.1007/s13399-025-06847-4
18.Papaefthimiou, M.; Kontou, P.I.; Bagos, P.G.; Braliou, G.G. Integration of Antioxidant Activity Assays Data of Stevia Leaf Extracts: A Systematic Review and Meta-Analysis. Antioxidants 2024; 13(6):692. https://doi.org/10.3390/antiox13060692
19.Andriopoulos, V.; Kornaros, M. Microalgal Phenolics: Systematic Review with a Focus on Methodological Assessment and Meta-Analysis. Mar. Drugs 2024; 22(10):460. https://doi.org/10.3390/md22100460
20.Sultana, S.; Lawag, I.L.; Lim, L.Y.; Foster, K.J.; Locher, C. A Critical Exploration of the Total Flavonoid Content Assay for Honey. Methods Protoc. 2024; 7(6):95. https://doi.org/10.3390/mps7060095
21.Sarma, N.; Upton, R.; Rose, U.; Guo, D.-a.; Marles, R.; Khan, I.; Giancaspro, G. Pharmacopeial Standards for the Quality Control of Botanical Dietary Supplements in the United States. J. Diet. Suppl. 2023; 20(3):485-504. https://doi.org/10.1080/19390211.2021.1990171
22.Sahraeian, S.; Rashidinejad, A.; Golmakani, M.-T. Recent advances in the conjugation approaches for enhancing the bioavailability of polyphenols. Food. Hydrocoll. 2024; 146:109221. https://doi.org/10.1016/j.foodhyd.2023.109221
23.Rocchetti, G.; Gregorio, R.P.; Lorenzo, J.M.; Barba, F.J.; Oliveira, P.G.; Prieto, M.A.; Simal-Gandara, J.; Mosele, J.I.; Motilva, M.-J.; Tomas, M., et al. Functional implications of bound phenolic compounds and phenolics–food interaction: A review. Compr. Rev. Food Saf. 2022; 21(2):811-842. https://doi.org/10.1111/1541-4337.12921
24.Evary, Y.M.; Amir, M.N.; Raihan, M.; Laela, D.P. Effects of Solvent Type and Simplicia-to-Solvent Ratio on the Phenolics, Flavonoids, and Antioxidant Activity of Torch Ginger (Etlingera elatior) Fruit. Trop. J. Nat. Prod. Res. 2025; 9(2):527-533. https://doi.org/10.26538/tjnpr/v9i2.16
25.Sakiroff, L.-M.; Chennell, P.; Yessaad, M.; Pereira, B.; Bouattour, Y.; Sautou, V. Evaluation of color changes during stability studies using spectrophotometric chromaticity measurements versus visual examination. Sci. Rep. 2022; 12(1):8959. https://doi/org/10.1038/s41598-022-13025-3
26.Beaver, K.; Dantanarayana, A.; Minteer, S.D. Materials Approaches for Improving Electrochemical Sensor Performance. J. Phys. Chem. B 2021; 125(43):11820-11834. https://doi.org/10.1021/acs.jpcb.1c07063
27.Vidal-Casanella, O.; Núñez, O.; Granados, M.; Saurina, J.; Sentellas, S. Analytical Methods for Exploring Nutraceuticals Based on Phenolic Acids and Polyphenols. Appl. Sci. 2021; 11(18):8276. https://doi.org/10.3390/app11188276
28.Abidin, Z.; Razak, R.; Pratama, M. Phytochemical Constituents, Antioxidant and Anti-inflammatory Potentials of Dechlorophyllized Extract of Kenikir Leaves (Cosmos caudatus Kunth). Trop. J. Nat. Prod. Res. 2025; 9(5). https://doi.org/10.26538/tjnpr/v9i5.11
29.Lama-Muñoz, A.; Contreras, M.d.M. Extraction Systems and Analytical Techniques for Food Phenolic Compounds: A Review. Foods 2022; 11(22):3671. https://doi.org/10.3390/foods11223671
30.Grondalska, J.; Kolniak-Ostek, J. Evaluation of Anti-Inflammatory, Antidiabetic, Antioxidant, and Anticholinergic Activities, as Well as Chemical Composition and Polyphenolic Compounds in Novel SCOBY-Fermented Juices. Molecules 2025; 30(9):1940. https://doi.org/10.3390/molecules30091940
31.Bibi, N.; Shah, M.H.; Khan, N.; Al-Hashimi, A.; Elshikh, M.S.; Iqbal, A.; Ahmad, S.; Abbasi, A.M. Variations in Total Phenolic, Total Flavonoid Contents, and Free Radicals’ Scavenging Potential of Onion Varieties Planted under Diverse Environmental Conditions. Plants 2022; 11(7):950. https://doi.org/10.3390/plants11070950
32.Okolie, N.P.; Falodun, A.; Davids, O. Evaluation of The Antioxidant Activity of Root Extract of Pepper Fruit (Dennetia Tripetala), and it’s Potential for the Inhibition of Lipid Peroxidation. Afr. J. Tradit. Complement. Altern. Med. 2014; 11(3):221-227. https://doi.org/10.4314/ajtcam.v11i3.31
33.Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J Food Compos Anal 2011; 24(7):1043-1048. https://doi.org/10.1016/j.jfca.2011.01.008
34.Iheanacho, C.M.; Akubuiro, P.C.; Oseghale, I.O.; Imieje, V.O.; Erharuyi, O.; Ogbeide, K.O.; Jideonwo, A.N.; Falodun, A. Evaluation of the Antioxidant Activity of the Stem Bark Extracts of Anacardium occidentale (Linn) Anacardiaceae. Trop. J. Phytochem. Pharm. Sci. 2023; 2(2):65-69. https://www.doi.org/10.26538/tjpps/v2i2.4
35.Egharevba, E.; Chukwuemeke-Nwani, P.; Eboh, U.; Okoye, E.; Bolanle, I.O.; Oseghale, I.O.; Imieje, V.O.; Erharuyi, O.; Falodun, A. Evaluation of the antioxidant and hypoglycaemic potentials of the leaf extracts of Stachytarphyta jamaicensis (Verbenaceae). Trop. J. Nat. Prod. Res. 2019; 3:170-174. https://doi.org/10.26538/tjnpr/v3i5.4