Biochemical and Immune Modulation in Mice Fed Defatted Black Soldier Fly (Hermetia illucens L.) Larvae Meal
Main Article Content
Abstract
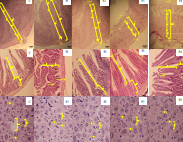
Black soldier fly larvae (BSFL; Hermetia illucens L.) have gained attention as a sustainable, high-protein natural product with potential applications in animal feed. Defatted BSFL meal, a byproduct of oil extraction, offers enhanced nutritional profiles while minimizing excess dietary fat. Despite its growing use, its effects on blood biochemistry, hematology, and tissue morphology in mammals remain underexplored. Addressing this gap, this study evaluated the impact of defatted BSFL meal supplementation (5%, 10%, 15%, and 20%) on growth, hematological, biochemical, and antioxidant parameters, and histology in mice over 30 days. Mice fed up to 15% BSFL supplementation showed no significant differences in red blood cell counts, hemoglobin, liver enzymes (Alanine Transaminase and Aspartate Transaminase), antioxidant enzymes (Superoxide Dismutase and Catalase), or Malondialdehyde content compared to controls. However, at 20% supplementation, white blood cell counts significantly increased (14.00×10³/µL vs. 8.95×10³/µL in control), suggesting mild immunostimulation. Histological evaluation revealed slight hepatic vacuolation and villus erosion at 20% BSFL, while lower BSFL levels maintained normal tissue architecture. Ventricular wall thickness and villus height were preserved at 5–15% BSFL supplementation but declined slightly at 20%. In conclusion, moderate inclusion of defatted BSFL meal supported stable physiological and biochemical parameters without inducing toxicity. These findings highlight the feasibility of utilizing defatted BSFL meal as an environmentally friendly, functional protein source in animal feed, contributing to sustainable nutrition and offering potential applications for both livestock and human health.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Nugroho RA, Aryani R, Hardi EH, Manurung H, Rudianto R, Jati WN. Fermented palm kernel waste with different sugars as substrate for black soldier fly larvae. GJESM. 2024; 10(2):503-516. DOI: https://doi.org/10.23917/bioeksperimen.v10i1.23015
2. Siddiqui S, Snoeck E, Tello A, Alles M, Fernando I, Saraswati Y, Rahayu T, Grover R, Ullah M, Ristow B. Manipulation of the black soldier fly larvae (Hermetia illucens; Diptera: Stratiomyidae) fatty acid profile through the substrate. J Insect Food Feed. 2022:1-20. DOI: https://doi.org/10.3920/JIFF2021.0162
3. Al-Qazzaz MFA, Ismail D, Akit H, Idris LH. Effect of using insect larvae meal as a complete protein source on quality and productivity characteristics of laying hens. Rev Bras Zootec. 2016; 45:518-523. DOI: https://doi.org/10.1590/s1806-92902016000900003
4. Liu S, Raheel Tariq M, Zhang Q, Wang H, Wang F, Zheng C, Li K, Zhuang Z, Wang L. Dietary Influence on Growth, Physicochemical Stability, and Antimicrobial Mechanisms of Antimicrobial Peptides in Black Soldier Fly Larvae. Insects. 2024; 15(11):872. DOI: https://doi.org/10.3390/insects15110872
5. Siddiqui SA, Ristow B, Rahayu T, Putra NS, Yuwono NW, Mategeko B, Smetana S, Saki M, Nawaz A, Nagdalian A. Black soldier fly larvae (BSFL) and their affinity for organic waste processing. Waste Manag. 2022; 140:1-13. DOI: https://doi.org/10.1016/j.wasman.2021.12.044
6. Gasco L, Oddon SB, Vandenberg G, Veldkamp T, Biasato I. Factors affecting the decision-making process of using insect-based products in animal feed formulations. 2023; 1:1-12. DOI: https://doi.org/10.3920/JIFF2022.0164
7. Rahayu R, Utari SD, Santoso P, Zaini E, Jessica A. Effectiveness of black soldier fly (Hermetia illucens) prepupa oil emulgel for burn wound recovery. Trop J Pharm Res. 2024; 8(3):6589-6593. DOI: https://doi.org/10.26538/tjnpr/v8i3.17
8. Srikha T, Pootthachaya P, Puangsap W, Pintaphrom N, Somparn N, Boonkum W, Cherdthong A, Tengjaroenkul B, Wongtangtintharn S. Effects of Black Soldier Fly Larvae Oil on Growth Performance, Blood Biochemical Parameters, Carcass Quality, and Metabolomics Profile of Breast Muscle of Thai Native Chickens. Animals. 2024; 14(21):3098. DOI: https://doi.org/10.3390/ani14213098
9. Bongiorno V, Gariglio M, Zambotto V, Cappone EE, Biasato I, Renna M, Forte C, Coudron C, Bergagna S, Gai F. Black soldier fly larvae used for environmental enrichment purposes: Can they affect the growth, slaughter performance, and blood chemistry of medium-growing chickens? Front Vet Sci. 2022; 9:1064017. DOI: https://doi.org/10.3389/fvets.2022.1064017
10. Priyadarshana MKC, Walpita CN, Ruwandeepika HAD, Magamage MPS. Effects of Black Soldier Fly, Hermetia illucens (Linnaeus, 1758), Larvae Incorporated Feed on Histomorphology, Gut Microbiota and Blood Chemistry of Cultured Fishes: A Review. Asian Fish Sci. 2022; 35(3). DOI: https://doi.org/10.33997/j.afs.2022.35.3.005
11. Yu Z, Sun Z, Ou B, Zhou M, Huang Y, Tan X. Effects of partial replacement of fish meal with black soldier fly (Hermetia illucens) larvae meal on growth performance, lipid metabolism and hepatointestinal health of juvenile golden pompano (Trachinotus ovatus). Aquacult Rep. 2023; 33:101824. DOI: https://doi.org/10.1016/j.aqrep.2023.101824
12. Huang W, Wang C, Chen Q, Chen F, Hu H, Li J, He Q, Yu X. Physicochemical, functional, and antioxidant properties of black soldier fly larvae protein. 2023; 89(1):259-275. DOI: https://doi.org/10.1111/1750-3841.16846
13. Aprianto MA, Kurniawati A, Hanim C, Ariyadi B, Al Anas M. Effect supplementation of black soldier fly larvae oil (Hermetia illucens L.) calcium salt on performance, blood biochemical profile, carcass characteristic, meat quality, and gene expression in fat metabolism broilers. Poult Sci. 2023; 102(10):102984. DOI: https://doi.org/10.1016/j.psj.2023.102984
14. Hoc B, Genva M, Fauconnier ML, Lognay G, Francis F, Caparros Megido R. About lipid metabolism in Hermetia illucens (L. 1758): on the origin of fatty acids in prepupae. Sci Rep. 2020; 10(1):11916. DOI: https://doi.org/10.1038/s41598-020-68784-8
15. Makkar HPS, Tran G, Heuzé V, Ankers P. State-of-the-art on use of insects as animal feed. AFST. 2014; 197:1-33.
16. Ameri A, Rahmati A, Soroushfar S, Lalehzari M, Dehghani T, Haghi-Aminjan H, Shamseddin J, Omidi M. The Protective Effect of N-acetylcysteine against Deltamethrin-Induced Hepatotoxicity in Mice. Avicenna J Med Biotechnol. 2024; 16(2):88-94. DOI: https://doi.org/10.18502/ajmb.v16i2.14859
17. Salahshoor M, Mohamadian S, Kakabaraei S, Roshankhah S, Jalili C. Curcumin improves liver damage in male mice exposed to nicotine. J Tradit Complement Med. 2016; 6(2):176-83. DOI: https://doi.org/10.1016/j.jtcme.2014.11.034
18. Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988; 34(3):497-500. DOI: https://doi.org/10.1093/clinchem/34.3.497
19. Feng T-Y, Li Q, Ren F, Xi H-M, Lv D-L, Li Y, Hu J-H. Melatonin Protects Goat Spermatogonial Stem Cells against Oxidative Damage during Cryopreservation by Improving Antioxidant Capacity and Inhibiting Mitochondrial Apoptosis Pathway. 2020; 2020(1):5954635. DOI: https://doi.org/10.1155/2020/5954635
20. Senthilkumar M, Amaresan N, Sankaranarayanan A. Estimation of Malondialdehyde (MDA) by Thiobarbituric Acid (TBA) Assay. Plant-Microbe Interactions: Laboratory Techniques. 2021:103-105. DOI: https://doi.org/10.1007/978-1-0716-1080-0_25
21. Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. 2008; 2008(5). DOI: https://doi.org/10.1101/pdb.prot4986
22. Kaiser F, Harbach H, Schulz C. Rapeseed proteins as fishmeal alternatives: A review. Rev Aquac. 2022; 14(4):1887-1911. DOI: https://doi.org/10.1111/raq.12678
23. Kondo Y, Aoki H, Masuda M, Nishi H, Noda Y, Hakuno F, Takahashi SI, Chiba T, Ishigami A. Moderate protein intake percentage in mice for maintaining metabolic health during approach to old age. Geroscience. 2023; 45(4):2707-2726. DOI: https://doi.org/10.1007/s11357-023-00797-3
24. Finke MD. Complete nutrient content of four species of commercially available feeder insects fed enhanced diets during growth. Zoo biology. 2015; 34(6):554-564. DOI: https://doi.org/10.1002/zoo.21246
25. Rumbos C, Rigopoulou M, Athanassiou C. Are insect meals prone to insect infestation during storage? Development of major storage insects on substrates based on Tenebrio molitor larvae meal. J Pest Sci. 2020; 93:1359-1367. DOI: https://doi.org/10.1007/s10340-020-01228-4
26. Van Huis A. Potential of insects as food and feed in assuring food security. Annu Rev Entomol. 2013; 58:563-583. DOI: https://doi.org/10.1146/annurev-ento-120811-153704
27. Schiavone A, Cullere M, De Marco M, Meneguz M, Biasato I, Bergagna S, Dezzutto D, Gai F, Dabbou S, Gasco L. Partial or total replacement of soybean oil by black soldier fly larvae (Hermetia illucens L.) fat in broiler diets: Effect on growth performances, feed-choice, blood traits, carcass characteristics and meat quality. Ital J Anim Sci. 2017; 16(1):93-100. DOI: https://doi.org/10.1080/1828051X.2016.1249968
28. Bian H, Qiao Y, Li Y, Wang Z, Zhao L, Li Z, Cheng B, Ding G. The Growth Performance and Nutrient Composition of Black Soldier Fly (Hermetia illucens) Larvae Fed Slaughtered Bovine Blood. Insects. 2024; 15(9):635. DOI: https://doi.org/10.3390/insects15090635
29. Rumpold BA, Schlüter OK. Potential and challenges of insects as an innovative source for food and feed production. Innov Food Sci Emerg Technol. 2013; 17:1-11. DOI: https://doi.org/10.1016/j.ifset.2012.11.005
30. Sawicka B, Umachandran K, Nasir NA-n, Skiba D. Alternative and new protein sources. Functional foods and nutraceuticals: bioactive components, formulations and innovations. 2020; 1(1):109-137. DOI: https://doi.org/10.1007/978-3-030-42319-3_7
31. Zhao J, Ban T, Miyawaki H, Hirayasu H, Izumo A, Iwase S-i, Kasai K, Kawasaki K. Long-term dietary fish meal substitution with the black soldier fly larval meal modifies the caecal microbiota and microbial pathway in laying hens. Animals. 2023; 13(16):2629. DOI: https://doi.org/10.3390/ani13162629
32. Fikri F, Purnomo A, Chhetri S, Purnama MTE, Çalışkan H. Effects of black soldier fly (Hermetia illucens) larvae meal on production performance, egg quality, and physiological properties in laying hens: A meta-analysis. Vet World. 2024; 17(8):1904. DOI: https://doi.org/10.14202/vetworld.2024.1904-1913
33. Li S, Ji H, Zhang B, Zhou J, Yu H. Defatted black soldier fly (Hermetia illucens) larvae meal in diets for juvenile Jian carp (Cyprinus carpio var. Jian): Growth performance, antioxidant enzyme activities, digestive enzyme activities, intestine and hepatopancreas histological structure. Aquaculture. 2017; 477:62-70. DOI: https://doi.org/10.1016/j.aquaculture.2017.04.015
34. Wang G, Peng K, Hu J, Mo W, Wei Z, Huang Y. Evaluation of defatted Hermetia illucens larvae meal for Litopenaeus vannamei: effects on growth performance, nutrition retention, antioxidant and immune response, digestive enzyme activity and hepatic morphology. Aquac Nutr. 2021; 27(4):986-997. DOI: https://doi.org/10.1111/anu.13240
35. Shin J, Lee K-J. Digestibility of insect meals for Pacific white shrimp (Litopenaeus vannamei) and their performance for growth, feed utilization and immune responses. PLoS One. 2021; 16(11):e0260305-e0260305. DOI: https://doi.org/10.1371/journal.pone.0260305
36. Chen X, Jin J, Hou F, Song B, Li Z, Zhao Y. Effects of black soldier fly larvae oil on growth performance, immunity and antioxidant capacity, and intestinal function and microbiota of broilers. J Appl Poult Res. 2022; 31(4):100292. DOI: https://doi.org/10.1016/j.japr.2022.100292
37. Makkar HP, Tran G, Heuzé V, Ankers P. State-of-the-art on use of insects as animal feed. Anim Feed Sci Technol. 2014; 197:1-33. DOI: https://doi.org/10.1016/j.anifeedsci.2014.07.008
38. Basili M, Randazzo B, Caccamo L, Guicciardi S, Meola M, Perdichizzi A, Quero GM, Maricchiolo G. Effect of Graded Inclusion of Black Soldier Fly (Hermetia Illucens, Linnaeus, 1758) Meal in Diets for Gilthead Seabream (Sparus aurata, Linnaeus, 1758) on Gut Microbiome and Liver Morphology. Res Sq. 2024; 2:1-39. DOI: https://doi.org/10.21203/rs.3.rs-4781211/v2
39. Wang T, Wang X, Shehata AI, Wang R, Yang H, Wang Y, Wang J, Zhang Z. Growth performance, physiological and antioxidant capacity responses to dietary fish meal replacement with insect meals for aquaculture: A case study in red claw crayfish (Cherax quadricarinatus). Aquacult Res. 2022; 53(10):3853-3864. DOI: https://doi.org/10.1111/are.15892
40. Zarantoniello M, Randazzo B, Secci G, Notarstefano V, Giorgini E, Lock E, Parisi G, Olivotto I. Application of laboratory methods for understanding fish responses to black soldier fly (Hermetia illucens) based diets. J Insect Food Feed. 2022; 8(11):1173-1195. DOI: https://doi.org/10.3920/JIFF2020.0135
41. Kim Y, Kim T, Choi Y, Lee J, Jang H, Kang M, Choi Y. Effects of hydrolysis and swelling on structural and functional properties of Hermetia illucens L.: insoluble protein in residue fraction. J Insect Food Feed. 2023; 9(6):799-807. DOI: https://doi.org/10.3920/JIFF2022.0128
42. Lo D, Loho L, Afandi FA, Romulo A, Tedjakusuma F, Somma T, Kaburuan ER, Zulkarnain A, Khatri-Chhetri R. Effect of different feed on nutritional content of black soldier fly (Hermetia illucens): A systematic review and meta-analysis. 2024; 1:66-78. DOI: https://doi.org/10.20956/canrea.v7i1.1153