Molecular Docking Prediction and In Vitro Investigation of Polyphenol Fraction from Senecio biafrae on Pro-Inflammatory Enzymes
Main Article Content
Abstract
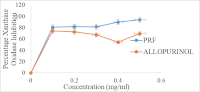
Plants containing anti-inflammatory compounds often regulate proinflammatory enzymes to prevent the onset of inflammatory conditions. This study aimed to examine the effect of Senecio biafrae leaf fractions on pro-inflammatory enzymes using an in vitro model, as well as molecular docking prediction technique to explore the anti-inflammatory properties of Senecio biafrae leaves. Thirty grams of Senecio biafrae crude extract were divided into two halves, with the first half partitioned and the second half fractionated using Amberlite XAD-16 resin to obtain polyphenol-rich fractions. The flavonoids and phenolic contents of the fractions were quantified. The polyphenol-rich fraction was fingerprinted using liquid chromatography mass spectrometry and evaluated for anti-proinflammatory enzyme activities. The compounds detected were subsequently analysed through molecular docking. The results indicated that the polyphenol-rich fraction had the highest total phenolic (1485.51±0.14 µg GAE/g) and flavonoid (428.07±0.03 µg QUE/g) concentrations. It also exhibited high xanthine oxidase activity in a dose-dependent manner and comparable lipoxygenase inhibitory activity with the standard anti-inflammatory drug. Comparing the polyphenol-rich fraction of Senecio biafrae to standard anti-inflammatory drugs, molecular docking analysis predicted that Fluperlapin and Methyl picraquassioside-A had the highest inhibitory activities against xanthine oxidase (-9.9 kcal/mol.) and cyclooxygenase (-8.9 kcal/mol.), while Stigmatellin Y had similar inhibitory activity against 5-lipoxygenase. Senecio biafrae's polyphenolic-rich fraction may serve as a foundation for the development of novel anti-inflammatory agents, but further research is needed to validate its anti-inflammatory properties through structural elucidation and in vivo investigation of the compounds identified in the polyphenol-rich fraction of Senecio biafrae.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.Karbab A, Mokhnache K, Ouhida S, Charef N, Djabi F, Arrar L, Mubarak MS. Anti-inflammatory, analgesic activity, and toxicity of Pituranthos scoparius stem extract: An ethnopharmacological study in rat and mouse models. J Ethnopharmacol. 2020; 258:112936.
2.Khelifi I, Hayouni EA, Cazaux S, Ksouri R, Bouajila J. Evaluation of in vitro biological activities: antioxidant; anti-inflammatory; anti-cholinesterase; anti-xanthine oxidase, anti-superoxyde dismutase, anti-α-glucosidase and cytotoxic of 19 bioflavonoids. Cell Mol Biol. 2020;66(1):9-19.
3.Ajiboye BO. Molecular interaction of bioactive compounds from Senecio biafrae leaf with α-amylase and α-glucosidase receptors. Clin Phyt. 2022;8(1):4.
4.Baiyeri S. Proximate, minerals, vitamins, and antinutrients and their correlations in the leaves of Senecio biafrae accessions. J Agric Sci (Belgrade). 2023;68(1):67-79.
5.Lienou LL, Telefo PB, Rodrigues GQ, Donfack JN, Araújo RA, Bruno JB, Njimou JR, Mbemya TG, Santos RR, Souza JF, Figueiredo JR. Effect of different extracts and fractions of Senecio biafrae (Oliv. & Hiern) J. Moore on in vivo and in vitro parameters of folliculogenesis in experimental animals. J ethnopharmacol. 2020; 251:112571.
6.JO A, OV O, JT A, OE A, AF A, OO B. Brine shrimps’ lethality test of ethanol extract and gas chromatography-mass spectrometry analysis of ethyl acetate fraction of Blighia sapida. Asian J Pharm Clin Res. 2020;13(8):208-212.
7.Sakulnarmrat K, Konczak I. Composition of native Australian herbs polyphenolic-rich fractions and in vitro inhibitory activities against key enzymes relevant to metabolic syndrome. Food Chem. 2012;134(2):1011-1119.
8.Singleton VL, Orthofer R, Lamuela-Raventos RM. Analysis of total phenols and other oxidation substrates and anti-oxidants by means of Folin-Ciocalteau reagent. Meth enzymol.1999;152-178.
9.Adeyemo AE, Omoba OS, Olagunju AI, Josiah SS. Assessment of nutritional values, phytochemical content, and antioxidant properties of Shallot (Allium ascalonicum L.) leaf and bulb. Measurement: Food. 2023; 10:1-9.
10.Adekola MB, Areola JO, Fagbohun OF, Asaolu FT, Ogundepo GE, Fajobi AO, Babalola OO. In-vitro antioxidant and anti-inflammatory activities of ethanol stem-bark extract of Blighia sapida KD Koenig. J Pharm Anal. 2022;12(2):350-354.
11.Sun PX, Yie LK, Zhang ZL, Hu M, Lu L. Colometric determination of the total content of the flavonoids in Epimedium capsules. J. Shenyang Pharm. Univ. 1999; 16:68-70.
12.Olarenwaju O, Apata JT, Akinpelu BO, Akomolafe RO, Oyemitan IA, Asaolu FT, Ologe MO, Iwalewa EO. Anti-inflammatory potentials, membrane stabilizing and xanthine oxidase inhibitory activities of Clerodendrum volibule ethanolic leaf extract on carragenaan-induced inflammation in rats. Int J Pharmacol Toxicol. 2018;6(1):7-11.
13.Nowacka-Jechalke N, Nowak R, Lemieszek MK, Rzeski W, Gawlik-Dziki U, Szpakowska N, Kaczyński Z. Promising potential of crude polysaccharides from Sparassis crispa against colon cancer: An in vitro study. Nutrients. 2021;13(1):161.
14.Lim MW, Yow YY, Gew LT. LC–MS profiling‐based non‐targeted secondary metabolite screening for deciphering cosmeceutical potential of Malaysian algae. J Cosmet Dermatol. 2023;22(10):2810-28
15.Johnson TO, Adegboyega AE, Iwaloye O, Eseola OA, Plass W, Afolabi B, Rotimi D, Ahmed EI, Albrakati A, Batiha GE, Adeyemi OS. Computational study of the therapeutic potentials of a new series of imidazole derivatives against SARS-CoV-2. J Pharmacol Sci. 2021;147(1): 62-71.
16.Bello O, Ayanda O, Aworunse O, Olukanmi B, Soladoye M, Esan E, Obembe O. Solanecio biafrae: An underutilized nutraceutically-important African indigenous vegetable. Pharmacog Rev. 2018;12(23): 128-132.
17.Zhuang S, Yun H, Zhou X, Li Y, Li S, Liu C, Zhang Y. Screening, isolation, and activity evaluation of potential xanthine oxidase inhibitors in Poria Cum Radix Pini and mechanism of action in the treatment of gout disease. J Sep Sci. 2024;47(1):2300505.
18.Kumar S, Chopra B, Dass R, Dhingra AK. Health Benefits of Nutraceuticals in Gout Patients. In nutraceuticals and bone health, Apple Acad Press, 2024, pp. 175-190.
19.Suchitha GP, Devasahayam Arokia Balaya R, Prasad TS, Dagamajalu S. A signaling network map of Lipoxin (LXA4): an anti-inflammatory molecule. Inflam Res. 2024;73(7): 1099-1106.
20.Costa V, Costa M, Videira RA, Andrade PB, Paiva-Martins F. Anti-inflammatory activity of olive oil polyphenols—The role of oleacein and its metabolites. Biomed. 2022;10(11):2990.
21.Galvis CE, Kouznetsov VV. Recent advances for the C–C and C–N bond formation in the synthesis of 1-phenethyl-tetrahydroisoquinoline, aporphine, homoaporphine, and β-carboline alkaloids. Syn. 2017;49(20): 4535-4561.
22.Talib WH, Baban MM, Azzam AO, Issa JJ, Ali AY, AlSuwais AK, Allala S, Al Kury LT. Allicin and Cancer Hallmarks. Mol. 2024;29(6):1320.
23.Zhang S, Mao B, Cui S, Zhang Q, Zhao J, Tang X, Chen W. Absorption, metabolism, bioactivity, and biotransformation of epigallocatechin gallate. Crit Rev Food Sci and Nutr. 2024;64(19): 6546-6566.
24.Nunes CD, Barreto Arantes M, Menezes de Faria Pereira S, Leandro da Cruz L, de Souza Passos M, Pereira de Moraes L, Vieira IJ, Barros de Oliveira D. Plants as sources of anti-inflammatory agents. Mol. 2020;25(16): 3726.
25.John A, Rusted J, Richards M, Gaysina D. Accumulation of affective symptoms and midlife cognitive function: the role of inflammation. Brain, Behav Immun. 2020; 84:164-172.
26.Mahboubi-Rabbani M, Abdolghaffari AH, Ghesmati M, Amini A, Zarghi A. Selective COX-2 inhibitors as anticancer agents: a patent review (2018-2023). Expt Opin Therap. Patents. 2024;34(9):733-757.
27.Szczukowski Ł, Krzyżak E, Zborowska A, Zając P, Potyrak K, Peregrym K, Wiatrak B, Marciniak A, Świątek P. Design, synthesis and comprehensive investigations of pyrrolo [3, 4-d] pyridazinone-based 1, 3, 4-oxadiazole as new class of selective cox-2 inhibitors. Int J Mol Sci. 2020;21(24): 9623.
28.Desind SZ, Bell SK, Davidson ZM, Lutz CS. Long noncoding RNAs and their complex role in shaping and regulating arachidonic acid metabolism: Learning to love the (not‐really) junk. Wiley Int Rev: RNA. 2024;15(1): e1828.
29.Wautier JL, Wautier MP. Pro-and anti-inflammatory prostaglandins and cytokines in humans: a mini review. Int J Mol Sci. 2023;24(11): 9647.
30.Kulkarni A, Nadler JL, Mirmira RG, Casimiro I. Regulation of tissue inflammation by 12-lipoxygenases. Biomol. 2021;11(5): 717.
31.Houglum JE, Harrelson GL, Seefeldt TM. Drugs for treating inflammation. In Principles of Pharmacology for Athletic Trainers, Routledge. 2024, pp. 96-123.
32.Karagiannis TC, Ververis K, Liang JJ, Pitsillou E, Kagarakis EA, Yi DT, Xu V, Hung A, El-Osta A. Investigation of the Anti-Inflammatory Properties of Bioactive Compounds from Olea europaea: In Silico Evaluation of Cyclooxygenase Enzyme Inhibition and Pharmacokinetic Profiling. Mol. 2024;29(15):3502.
33.Ramsis T, Selim HM, Elseedy H, Fayed EA. The role of current synthetic and possible plant and marine phytochemical compounds in the treatment of acne. RSC Adv. 2024;14(33):24287-24321.
34.Apalowo RK, Chronopoulos D. A wave-based numerical scheme for damage detection and identification in two-dimensional composite structures. Comp str. 2019; 214:164-182.
35.Moreira J, Ribeiro D, Silva PM, Nazareth N, Monteiro M, Palmeira A, Saraiva L, Pinto M, Bousbaa H, Cidade H. New alkoxy flavone derivatives targeting caspases: Synthesis and antitumor activity evaluation. Mol. 2018;24(1):129.