Ex-vivo Assessment of Mango (Mangifera indica) and Cashew (Anacardium occidentale) Leaf Extracts on Male Fertility: Impact on Sperm Parameters
Main Article Content
Abstract
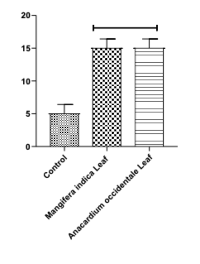
Rapid population growth challenges developing nations. Mango (Mangifera indica) and Cashew (Anacardium occidentale) leaves, known for antimalarial effects, demonstrate spermicidal potential, suggesting natural fertility control options amid rising reproductive health concerns and limited access to conventional contraceptives. Hence, this study looked into the in vitro potential of the aqueous extracts of M. indica and A. occidentale leaves on sperm concentration, sperm motility, and sperm morphology. The extracts were prepared by dissolving 0.5 g of the dried leaf powder in 100 mL of distilled water. The aqueous extracts of the herbs (10 μL) were added directly to 10 μL of sperm suspension of the sperm collected from adult male albino rats. The spermicidal effects of the extracts were observed using a microscope and compared to those of normal control sperm at the same volume ratio. The results showed that Mangifera indica significantly reduced sperm concentration and sperm motility, with p-values of p < 0.01 and p < 0.001, respectively. Anacardium occidentale leaves extracts significantly (p < 0.01) reduced sperm motility, with both extracts exerting varying effects on sperm morphology. Findings from this study revealed that Mangifera indica and Anacardium occidentale leaf extracts not only reduced sperm concentration and motility but also induced morphological abnormalities in spermatozoa (Head, neck, and tail sperm defects) when compared to the normal control sperm. This indicates that the studied leaves exhibit spermicidal effects, highlighting their potential use as natural agents for male fertility control.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Liu DH, Raftery AE. How do education and family planning accelerate fertility decline? Popul Dev Rev. 2020;46(3):409-441.
2. Baranon MD, Nahini AT, Ayena C, Soglo AM, Kollie TC. Spatio-temporal trends and patterns of synthetic fertility index across African countries: A comprehensive analysis from 1950 to 2023. East Afr J Interdiscip Stud. 2024;7(1):89-105.
3. Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. In: Clinical Biochemistry. 2018;62:2–10.
4. Ma M, Kim CH, Hall K, Kim JG. It takes two to avoid pregnancy: Addressing conflicting perceptions of birth control pill responsibility in romantic relationships. In: Proceedings of the ACM on Human-Computer Interaction. 2023;7(CSCW2):1–27.
5. Krapf JM, Goldstein AT. Combined estrogen-progestin oral contraceptives and female sexuality: an updated review. In: Sex Med Rev. 2024;12(3):307–320.
6. Verma N, Cwiak C, Kaunitz AM. Hormonal contraception: systemic estrogen and progestin preparations. In: Clinical Obstetrics and Gynaecology. 2021;64(4):721–738.
7. Monterrosa-Castro A, Redondo-Mendoza V, Monterrosa-Blanco A. Current knowledge of progestin-only pills. Electron J Gen Med. 2021;18(6):em 320.
8. Bastianelli C, Farris M, Bruni V, Rosato E, Brosens I, Benagiano G. Effects of progestin-only contraceptives on the endometrium. Expert Rev Clin Pharmacol. 2020;13(10):1103-1123.
9. Prager S, Steinauer J. Hormonal contraception. In: Reproductive Endocrinology and Infertility. CRC Press. 2024;65–83.
10. Schickler R, Patel J. Barrier contraceptives. In: The Handbook of Contraception: Evidence-Based Practice Recommendations and Rationales. 2020;163-181.
11. Mazer-Amirshahi M, Ye P. Emergency contraception in the emergency department. Am J Emerg Med. 2023;63:102-105.
12. Rashid S, Majeed LR, Nisar B, Nisar H, Bhat AA, Ganai BA. Phytomedicines: Diversity, extraction, and conservation strategies. In: Phytomedicine. Academic Press; 2021. p. 1-33.
13. El-Saadony MT, Zabermawi NM, Burollus MA, Shafi ME, Alagawany M, Abd El-Hack ME. Nutritional aspects and health benefits of bioactive plant compounds against infectious diseases: a review. Food Rev Int. 2023;39(4):2138-2160.
14. Noh S, Go A, Kim DB, Park M, Jeon HW, Kim B. Role of antioxidant natural products in the management of infertility: a review of their medicinal potential. Antioxidants. 2020;9(10):957.
15. Shunnarah A, Tumlinson R, Calderón AI. Natural products with potential for nonhormonal male contraception. J Nat Prod. 2021;84(10):2762-2774.
16. Haddad LB, Townsend JW, Sitruk-Ware R. Contraceptive technologies: looking ahead to new approaches to increase options for family planning. Clin Obstet Gynecol. 2021;64(3):435-448.
17. Nickels L, Yan W. Nonhormonal male contraceptive development—strategies for progress. Pharmacol Rev. 2024;76(1):37-48.
18. Ogbomade RS, Chike CPR, Adienbo OM. Evaluation of the anti-infertility effect of aqueous extract of Phyllanthus amarus in male Wistar rats. Exp. 2014;27(3):1874-1879.
19. AlSheikh HMA, Sultan I, Kumar V, Rather IA, Al-Sheikh H, Jan AT, Haq QMR. Plant-based phytochemicals as possible alternatives to antibiotics in combating bacterial drug resistance. Antibiotics. 2020;9(8):480.
20. Sharma D, Gupta S, Kumar R, Singh P, Singh A, Khan H. An ethnopharmacological, phytochemical, and pharmacological review of Mangifera indica (Mango). Res J Pharmacol Pharmacodyn. 2024;16(1):30-34.
21. Asanga EE, Okoroiwu H, Edet UO, Amaechi D, Nelson PE, Uchenwa M, M., Eseyin, OA, Samuel G, Ettah LA, Obongha OA. Antimalarial activity of Mangifera indica aqueous extract in Plasmodium berghei’s apicoplast. Trop J Pharm Res. 2023;22(5):1007-1015.
22. Zaffran VD. Role of glycosylation in immunoreactivity of major cashew (Anacardium occidentale L.) allergen, Ana O 1. The Florida State University. 2020.
23. Edet PI, Samuel HS. A review of antioxidant applications and phytochemical constituents of Anacardium occidentale leaf extract. Fac Nat Appl Sci J Sci Innov. 2023;5(1):15-20.
24. Iheanacho CM, Akubuiro PC, Oseghale IO, Imieje VO, Erharuyi O, Ogbeide KO, Jideonwo AN, Falodun A. Evaluation of the antioxidant activity of the stem bark extracts of Anacardium occidentale (Linn) Anacardiaceae. Trop J Phytochem Pharm Sci. 2023;2(2):65–69. Available from: http://www.doi.org/10.26538/tjpps/v2i2.4
25. Shodehinde S, Adefegha SA, Oboh G, Oyeleye SI, Olasehinde TA, Nwanna EE, Adedayo BC, Boligon AA. Phenolic composition and evaluation of methanol and aqueous extracts of bitter gourd (Momordica charantia L.) leaves on angiotensin-I-converting enzyme and some pro-oxidant-induced lipid peroxidation in vitro. J Evid Based Complement Altern Med. 2016;4:67-76. https://doi.org/10.1177/2156587216636505.
26. Ahmed Z. Analysis of phytochemical potentiality and in vitro antimicrobial properties of jute leaf extracts. Environ Sci. 2023;2(2):122.
27. Balkrishna A, Shankar R, Joshi RA, Joshi M, Prajapati UB, Srivastava A, Arya VP. The nutraceutical studies of the berries of Solanum violaceum Ortega, a traditional vegetable. J Pharmacogn Phytochem. 2024;13(4):136-141.
28. Hassanbeiki M, Golestan L, Mashak Z, Ahmadi M, Jafari SM. Production of a functional confectionary cream containing licorice root extract and double-coated Lactobacillus plantarum by alginate and malva mucilage. Carbohydr Polym Technol Appl. 2024;7:100435.
29. Saha S, Imran IB. Isolation, detection, and quantification of hydrolyzable tannins of the biosynthetic pathway by liquid chromatography coupled with tandem mass spectrometry. Rapid Commun Mass Spectrom. 2020;34(5):e9005. https://doi.org/10.1002/rcm.9005.
30. Patle TK, Shrivas K, Kurrey R, Upadhyay S, Jangde R, Chauhan R. Phytochemical screening and determination of phenolics and flavonoids in Dillenia pentagyna using UV-vis and FTIR spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc. 2020;242:118717.
31. Elezabeth DV, Subramanian A. Identification of phytochemical constituents and antimicrobial activity of Indigofera suffruticosa leaves. Int J Curr. 2013;1(7):6-10.
32. Leko BJ, Olawuyi ST, Okon LU. The mitigating effect of Ananas comosus on aluminium-induced oxidative stress on the testes of adult male Wistar rats. J Basic Appl Zool. 2021;82:1-12.
33. Nwanna EE, Inumisan PD, Olawuyi TS, Oboh G. Assessment of sperm quality in Plasmodium berghei NK65-infected mice treated with brimstone (Morinda lucida Benth) tree plant. Sci Afr. 2023;20:e01625.
34. Iliyasu D, Mustapha AR, Abdullahi MA, Abba A, Asuku SO, Rwuaan JS, Nwannenna AI. Significance of graded doses of aqueous seed extract of Moringa oleifera (L) on live body weight, gonadal, extragonadal dimensions, and sperm reserves of Yankasa rams. J Sustain Vet allied Sci. 2021;1(1):33–40.
35. Oluwatunase GO, Otulana OJ, Olusola OO, Fakunle BP, Royhaan F, Enemali FU, Dare SS. Sub-acute toxic effects of methanol root extract of Carpolobia alba G. Don on the testes of adult Wistar rats. J Exp Clin Anat. 2024;21(2):180–187.
36. Hassan AH, Banchi P, Domain G, El Khoury R, Chaaya R, Wydooghe E, Van Soom A. A comparative study of canine epididymal sperm collection techniques and cryopreservation. Front Vet Sci. 2023;10:1181054.
37. Mortimer ST, Mortimer D. Manual methods for sperm motility assessment. In: Methods in Molecular Biology. Springer; 2012. p. 61-75.
38. Chakraborty S, Saha S. Understanding sperm motility mechanisms and the implication of sperm surface molecules in promoting motility. Middle East Fertil Soc J. 2022;27(4). https://doi.org/10.1186/s43043-022-00094-7.
39. Olawuyi TS, Akinola BK, Ukwenya VO, Ayanda O. Histomorphology, hormonal changes and redox imbalance in aluminium-induced testicular toxicity: The mitigating influence of ethanolic stembark extract of Prosopis africana. Int J Innov Sci Res Technol. 2022;7(2).
40. Tanga BM, Qamar AY, Raza S, Bang S, Fang X, Yoon K, Cho J. Semen evaluation: methodological advancements in sperm quality-specific fertility assessment - A review. Anim Biosci. 2021;34(8):1253-1270. https://doi.org/10.5713/ab.21.0072.
41. Ambar RF, Maziotis E, Simopoulou M. Sperm concentration and total sperm count. In: Human Semen Analysis: From the WHO Manual to the Clinical Management of Infertile Men. Cham: Springer; 2024. p. 31-60.
42. Johnson HL, Lee SH. Terpenoids and male reproductive health: Effects on sperm parameters and testosterone levels. Reprod Toxicol. 2021;29(4):245-255. https://doi.org/10.1016/j.reprotox.2021.07.005.
43. Kumar M, Saurabh V, Tomar M, Hasan M, Changan S, Sasi M, Maheshwari C, Prajapati U, Singh S, Prajapat RK, Dhumal S. Mango (Mangifera indica L.) leaves: Nutritional composition, phytochemical profile, and health-promoting bioactivities. Antioxidants. 2021;10(2):299.
44. Evgeni E, Kothari P. Sperm motility. In: Human Semen Analysis: From the WHO Manual to the Clinical Management of Infertile Men. Cham: Springer; 2024. p. 61-101.
45. Tootian Z, Fazelipour S, Goodarzi N, Arab HA. The effect of pure phenol on sperm parameters and fertility rate in male mice. Iran J Vet Med. 2016;9(4):1-8. https://doi.org/10.1007/s11356-016-7960-y.
46. Saha P, Majumdar S, Pal D, Pal BC, Kabir SN. Evaluation of spermicidal activity of MI-saponin A. Reprod Sci. 2010;17(5):454-464.
47. Souad K, Ali S, Mounir A, Mounir TM. The spermicidal activity of the extract from Cestrum parqui. Contraception. 2007;75(2):152-156.
48. Capone S, Forleo A, Radogna AV, Longo V, My G, Genga A, Ferramosca A, Grassi G, Casino F, Siciliano P, Notari T. Innovative approach for human semen quality assessment based on volatilomics. Toxics. 2024;12(8):543.
49. Zhou B, Qiu Z, Liu G, Liu C, Zhang J. Spermicidal and anti-gonococcal effects of tannins from pomegranate rind. J Med. Plants Res. 2012;6(7):1334-1339.
50. Mishra R, Nikam A, Hiwarkar J, Nandgude T, Bayas J, Polshettiwar S. Flavonoids as potential therapeutics in male reproductive disorders. Future J Pharm Sci. 2024;10(1):100-110. https://doi.org/10.1186/s43094-024-00677-3.