Camel Milk Attenuates Cardiotoxicity Induced by Monosodium Glutamate via Inhibition of Oxidative Stress and Inflammation in Rats
Main Article Content
Abstract
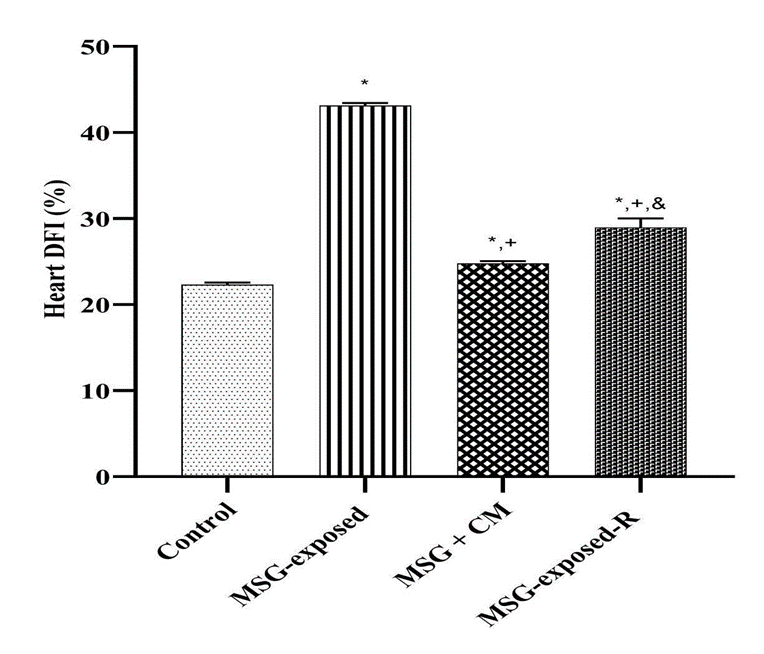
Monosodium glutamate (MSG), a widely used food additive, has been implicated in cardiotoxic effects through mechanisms involving oxidative stress, inflammation, and apoptosis. Camel milk (CM), known for its antioxidant and anti-inflammatory properties, may offer protective effects against such toxicity. This study evaluated the cardioprotective potential of CM against MSG-induced cardiac damage in rats. Forty male Wistar rats (185-205 g) were randomized into four groups (n = 10): Control (distilled water), MSG (6 g/kg), MSG + CM (MSG 6 g/kg + CM 5 ml/kg), and Recovery (MSG 6 g/kg followed by 21 days without CM treatment). All treatments were administered orally for 21 days. Body and heart weights were recorded. Cardiac tissues were assayed for myeloperoxidase (MPO), nitric oxide (NO), C-reactive protein (CRP), interleukin-1β (IL-1β), tumor necrosis factor-α (TNF-α), NF-κB, malondialdehyde (MDA), catalase (CAT), superoxide dismutase (SOD), reduced glutathione (GSH), glutathione-S-transferase (GST), glutathione peroxidase (GPx), caspase-3 activity, and DNA fragmentation index. Histopathological evaluation of the myocardium was performed. Data were analyzed by one-way ANOVA with Tukey's post hoc test (p < 0.05). MSG significantly increased inflammatory (MPO, NO, CRP, IL-1β, TNF-α, NF-κB), oxidative (MDA), apoptotic (caspase-3), and DNA damage markers, while reducing antioxidant enzyme activities (CAT, SOD, GSH, GST, GPx) and heart weight (p < 0.05). CM restored antioxidant defenses, lowered inflammatory and apoptotic indices, reduced DNA fragmentation, and preserved myocardial architecture. The recovery group exhibited partial but less pronounced improvements. Camel milk effectively mitigates MSG-induced cardiotoxicity, highlighting its importance as a natural cardioprotective dietary intervention.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.Hazzaa SM, El-Roghy ES, Abd Eldaim MA, Elgarawany GE. Monosodium glutamate induces cardiac toxicity via oxidative stress, fibrosis, and P53 proapoptotic protein expression in rats. Environ Sci Pollut Res. 2020;27:20014–20024.
2.Thuy LTP, Phuong TT, Nhung BTT. Monosodium glutamate: A review of potential health risks. Viet J Sci Technol. 2020;58(3A):45–53.
3.Tracy S. Monosodium Glutamate: A Global Food Additive. Food Hist J. 2016;12(1):35–47.
4.Krynytska I, Maruniak E, Tokar O. The influence of monosodium glutamate on liver and heart tissues in rats. Ukr Biochem J. 2019;91(2):68–76.
5.Aimo A, Castiglione V, Borrelli C, Saccaro LF, Franzini M, Masi S, Emdin M, Giannoni A. Oxidative stress and inflammation in the evolution of heart failure: From pathophysiology to therapeutic strategies. Eur J Prev Cardiol. 2020;27(5):494–510.
6.Hassan HA, El Agamy DS, Abdel-Rahman RF. Neuroprotective effects of ellagic acid against MSG-induced neurotoxicity via inhibition of oxidative stress and apoptosis. Environ Sci Pollut Res. 2020;27(26):32913–32924.
7.Souliotis VL, Vlachogiannis NI, Pappa M, Argyriou A, Ntouros PA, Sfikakis PP. DNA damage response and oxidative stress in systemic autoimmunity. Int J of Mol Sci. 2019 20;21(1):55.
8.Abrhaley A, Leta S. Medicinal values of camel milk. Int J Vet Sci Res. 2018;4(2):35–40.
9.Behrouz S, Saadat S, Memarzia A, Sarir H, Folkerts G, Boskabady MH. The antioxidant, anti-inflammatory and immunomodulatory effects of camel milk. Front in immun. 2022 12;13:855342
10.Nalugo H, Ninsiima HI, Kasozi KI, Nabirumbi R, Osuwat LO, Matama K, Ssempijja F, Batiha GE. Monosodium Glutamate Maintains Antioxidant Balance in the Neuro-Retinal Axis of Male Wistar Rats.
11.Abubakar AL, Dandare A, Dandare SU, Rabiu S, Ibrahim AS, Armaya’u S. Effect of camel milk supplementation in the management of gastric ulcers. Appl Med Res. 2018;4(1):12–17.
12.Ajayi AF, Akhigbe RE. Staging of the estrous cycle and induction of estrus in experimental rodents: an update. Fertil Res Pract. 2020;6:1–15.
13.Zaidi AS, Muzaffar M, Gautam S, Alam I. Effect of curcumin on inflammatory and oxidative stress markers in lipopolysaccharide-induced animal models of pre-eclampsia. Physiology. 2024;39(S1):1737.
14.Varthya SB, Sarma P, Bhatia A, Shekhar N, Prajapat M, Kaur H, Thangaraju P, Kumar S, Singh R, Siingh A, Prakash A. Efficacy of green tea, its polyphenols, and nanoformulation in experimental colitis and the role of NF-kB pathway: a preclinical in-vivo and in-silico exploratory study. J Biomol Struct Dyn. 2021;39(14):5314–5326.
15.Yucel AA, Gulen S, Dincer S, Yucel AE, Yetkin GI. Comparison of two different applications of the Griess method for nitric oxide measurement. J Exp Integr Med. 2012;2(1):167.
16.Seven E, Husemoen LL, Sehested TS, Ibsen H, Wachtell K, Linneberg A, Jeppesen JL. Adipocytokines, C-reactive protein, and cardiovascular disease: a population-based prospective study. PLoS One. 2015;10(6):e0128987.
17.Hira K, Sajeli Begum A. Methods for evaluation of the TNF-α inhibition effect. In: The TNF Superfamily: Methods and Protocols. New York, NY: Springer US; 2020. p. 271–279.
18.Çetin A, Şen A, Çetin I, Çimen B, Cimen L, Savas G, Öztürk A, Koker MY. Comparison of ELISA and flow cytometry for measurement of interleukin-1 beta, interleukin-6, and tumor necrosis factor-α. Turk J Biochem. 2018;43(5):540–548.
19.Shi G, Li D, Fu J, Sun Y, Li Y, Qu R, Jin X, Li D. Upregulation of cyclooxygenase-2 is associated with activation of the alternative nuclear factor kappa B signaling pathway in colonic adenocarcinoma. Am J Transl Res. 2015;7(9):1612.
20.Ghani MA, Barril C, Bedgood DR Jr, Prenzler PD. Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay. Food Chem. 2017;230:195–207.
21.Vuolo MM, da Silva-Maia JK, Batista ÂG. The GSH colorimetric method as a measurement of antioxidant status in serum and rodent tissues. In: Basic Protocols in Foods and Nutrition. New York, NY: Springer US; 2022. p. 187–194.
22.Hadwan MH, Kadhum Ali S. New spectrophotometric assay for assessments of catalase activity in biological samples. Anal Biochem. 2018;542:29–33.
23.Ahmed AY, Aowda SA, Hadwan MH. A validated method to assess glutathione peroxidase enzyme activity. Chem Pap. 2021;75:6625–6633.
24.Dada A, da Silva RD, Zanovello M, Moser JC, Orengo SL, Cavichiolo MO, Bidinha ER, Boeing T, Cechinel-Filho V, de Souza P. Comparative Analysis of the Protective Effect of Naringenin on Cardiovascular Parameters of Normotensive and Hypertensive Rats Subjected to the Myocardial Infarction Model. Pharmaceuticals. 2024 4;17(10):1324.
25.Choudhary GS, Al-Harbi S, Almasan A. Caspase-3 activation is a critical determinant of genotoxic stress-induced apoptosis. In: Apoptosis and Cancer: Methods and Protocols. 2015. p. 1–9.
26.Kim JH, Lee JS, Park HJ. A quantitative diphenylamine assay for DNA fragmentation in myocardial infarction. J Mol Cell Cardiol. 2016;94:1–7.
27.Sridharan D, Pracha N, Dougherty JA, Akhtar A, Alvi SB, Khan M. A. One-stop protocol to assess myocardial fibrosis in frozen and paraffin sections. Methods Protoc. 2022;5(1):13.
28.Akataobi US. Effect of monosodium glutamate (MSG) on behavior, body, and brain weights of exposed rats. Environ Dis. 2020;5(1):3–8.
29.Arain MA, Khaskheli GB, Shah AH, Marghazani IB, Barham GS, Shah QA, Khand FM, Buzdar JA, Soomro F, Fazlani SA Nutritional significance and promising therapeutic/medicinal application of camel milk as a functional food in humans and animals: A comprehensive review. Anim Biotechnol. 2023;34(6).
30.Hamed H, Gargouri M, Bellassoued K, Ghannoudi Z, Elfeki A, Gargouri A. Cardiopreventive effects of camel milk against carbon tetrachloride-induced oxidative stress, biochemical and histological alterations in mice. Arch Physiol Biochem. 2018;124(3):253–260.
31.Koziarska-Rościszewska M, Gluba-Brzózka A, Franczyk B, Rysz J. High-sensitivity C-reactive protein relationship with metabolic disorders and cardiovascular diseases risk factors. Life. 2021 11(8):742.
32.Asejeje FO, Gabriel GO, Abiola MA. Monosodium glutamate aggravates lipopolysaccharide-induced liver injury via inflammation and oxidative stress in rats. Nutrire. 2023;48(1):5.
33.Zanfirescu A, Ungurianu A, Tsatsakis AM, Nițulescu GM, Kouretas D, Veskoukis A, Tsoukalas D, Engin AB, Aschner M, Margină D. A review of the alleged health hazards of monosodium glutamate. Compr Rev Food Sci Food Saf. 2019;18(4):1111–1134.
34.El-Bahr SM, Alnahas AA, Zabady MK. Camel milk modulates lipid metabolism, expression of enzymatic antioxidant genes, and paraoxonase activity in rats fed a high-cholesterol diet. Slov Vet Res. 2023;60.