Triterpenoids from the Stems of Nauclea orientalis (L.) L. (Rubiaceae)
Main Article Content
Abstract
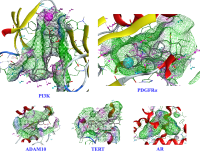
Nauclea orientalis (L.) L. is a well-known source of varied secondary metabolites that underlie its traditional therapeutic uses and pharmacological activity. Studies on this plant in Vietnam have identified various compounds, but an investigation into the triterpenoids from its Vietnamese species and their specific biological activities has not been thoroughly conducted. This study focuses on the isolation, structural elucidation, and bioactivity evaluation of triterpenoids from the chloroform-soluble fraction of Nauclea orientalis stems. Through combined chromatographic methods, four triterpenoids (1–4) were successfully isolated. These compounds were identified as 3β,6β,23-trihydroxyolean-12-en-28-oic acid (1), rotundic acid 3,23-acetonide (2), oleanolic acid (3), and ursolic acid (4), based on spectroscopic analysis and comparison with literature data. Notably, a complete comprehensive NMR dataset, including 1H, 13C, HSQC, HMBC, and NOESY correlations, was provided for compound 1. This analysis confirmed its relative stereochemistry, resolving ambiguities present in previous literature reports. All isolated compounds were evaluated for cytotoxicity against the human breast cancer MCF-7 cell line using an MTT assay. Compound 1 exhibited the most promising moderate cytotoxicity with an IC50 value of 34.17 μM (48 h incubation), while compound 3 showed an IC50 of 87.63 μM (24 h incubation). In silico target prediction and molecular docking studies identified compound 1 as a promising hit, binding to several breast cancer-related targets (e.g., PI3K, PDGFRα). Pharmacophore modeling further suggested that the C-23 hydroxy group is a prime target for structural modification to enhance potency. These findings highlight compound 1 as a valuable scaffold for designing new cytotoxic derivatives.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Bin L, Qi G, Zhiwen C, Li L, Peipei L, Lin L, Lan Y and Cheng L. Nauclea officinalis: A Chinese medicinal herb with phytochemical, biological, and pharmacological effects. Chin Med. 2022; 17: 141. doi:10.1186/s13020-022-00691-8.
2. Songoen W, Brunmair J, Traxler F, Wieser VC, Phanchai W, Pluempanupat W, Brecker L and Schinnerl J. Yellow twig (Nauclea orientalis) from Thailand: Strictosamide as the key alkaloid of this plant species. Molecules. 2022; 27(16): 5176. doi:10.3390/molecules27165176.
3. Fujita E, Fujita T and Suzuki T. On the constituents of Nauclea orientalis L. I. Noreugenin and naucleoside, a new glycoside. (Terpenoids V). Chem Pharm Bull. 1967; 15(11): 1682-1686. doi:10.1248/cpb.15.1682.
4. Liu YP, Peng-Kun J, Jin-Tao L, Liang L, Wan-Hui Z, Chao Z, Zhi-Jie Z and Fu YH. Cytotoxic indole alkaloids from Nauclea orientalis. Nat Prod Res. 2018; 32(24): 2922-2927. doi:10.1080/14786419.2017.1395429.
5. Zhang Z, ElSohly HN, Jacob MR, Pasco DS, Walker LA and Clark AM. New indole alkaloids from the bark of Nauclea orientalis. J Nat Prod. 2001; 64(8): 1001-1005. doi:10.1021/np010042g.
6. Sichaem J, Surapinit S, Siripong P, Khumkratok S, Jong-aramruang J and Tip-pyang S. Two new cytotoxic isomeric indole alkaloids from the roots of Nauclea orientalis. Fitoterapia. 2010; 81(7): 830-833. doi:10.1016/j.fitote.2010.05.004.
7. Erdelmeier CA, Regenass U, Rali T and Sticher O. Indole alkaloids with in vitro antiproliferative activity from the ammoniacal extract of Nauclea orientalis. Planta Med. 1992; 58(01): 43-48. doi:10.1055/s-2006-961387.
8. Sandamali JAN, Hewawasam RP, Jayatilaka KAPW and Mudduwa LKB. Nauclea orientalis (L.) bark extract protects rat cardiomyocytes from doxorubicin-induced oxidative stress, inflammation, apoptosis, and DNA fragmentation. Oxid Med Cell Longev. 2022; 2022(1): 1714841. doi:10.1155/2022/1714841.
9. Nia K, Reny DN, and Lestyo W. Antibacterial activity of gempol (Nauclea orientalis L.) leaf ethanolic extract and its fractions against Escherichia coli and Staphylococcus aureus. Sci J Pharm. 2022; 18(1): 1-12. doi:10.20885/jif.vol18.iss1.art1.
10. Dao PTA, Le QT and Mai NTT. Constituents of the stem of Nauclea orientalis. Nat Prod Commun. 2015; 10(11): 1934578X1501001122. doi:10.1177/1934578X1501001122.
11. Bouzeko ILT, Dongmo FLM, Ndontsa BL, Ngansop CAN, Keumoe R, Bitchagno GTM, Jouda JB, Mbouangouere R, Tchegnitegni BT, Boyom FF, Sewald N, Lenta BN, Tane P, Ngouela SA and Tene M, Chemical constituents of Mussaenda erythrophylla Schumach. & Thonn. (Rubiaceae) and their chemophenetic significance. Biochem Syst Ecol. 2021; 98: 104329. doi: 10.1016/j.bse.2021.104329.
12. John VA, Enoch IA, John I and Terrumun ATA. Isolation and characterisation of bioactive principles from sapwood of Pterocarpus santalinoides. Trop J Nat Prod Res. 2025; 9(7): 3214-3224. doi: 10.26538/tjnpr/v9i7.49.
13. Denitsa KK, Milena TN and Ina YA. Chemical profiling and antioxidant capacity assessment of three endemic thymus species distributed in Bulgaria. Trop J Nat Prod Res. 2025; 9(2): 487-494. doi: 10.26538/tjnpr/v9i2.11.
14. Khan IA, Sticher O and Rali T. New triterpenes from the leaves of Timonius timon. J Nat Prod. 1993; 56(12): 2163-2165. doi:10.1021/np50102a019.
15. Yu QL, Duan HQ, Takaishi Y and Gao WY. A novel triterpene from Centella asiatica. Molecules. 2006; 11(9): 661-665. doi:10.3390/11090661.
16. Quang TH, Ngan NTT, Minh CV, Kiem PV, Boo HJ, Hyun JW, Kang HK and Kim YH. Cytotoxic triterpene saponins from the stem bark of Kalopanax pictus. Phytochem Lett. 2012; 5(1): 177-182. doi:10.1016/j.phytol.2011.12.005.
17. Luo Y, Xu QL, Dong LM, Zhou ZY, Chen YC, Zhang WM and Tan JW. A new ursane and a new oleanane triterpene acids from the whole plant of Spermacoce latifolia. Phytochem Lett. 2015; 11: 127-131. doi:10.1016/j.phytol.2014.12.005.
18. Bankeu JJK, Kagho DUK, Fongang YSF, Toghueo RMK, Mba’ning BM, Feuya GRT, Fekam FB, Tchouankeu JC, Ngouela SA, Sewald N, Lenta BN and Ali MS. Constituents from Nauclea latifolia with anti-haemophilus influenzae type B inhibitory activities. J Nat Prod. 2019; 82(9): 2580-2585. doi:10.1021/acs.jnatprod.9b00463.
19. Cuong LCV, Tuan AL, That HDT, Phuong ATT, Quynh LL, Ho KY and Anh HLT. Cytotoxic and anti-inflammatory activities of secondary metabolites from Ophiorrhiza baviensis growing in Thua Thien Hue, Vietnam. Nat Prod Res. 2021; 35(22): 4218-4224. doi:10.1080/14786419.2019.1693564.
20. Tri MD, Phat NT, Minh PN, Chi MT, Hao BX, Minh An TN, Alam M, Kieu NV, Dang VS, Mai TTN and Duong TH. In vitro anti-inflammatory, in silico molecular docking and molecular dynamics simulation of oleanane-type triterpenes from aerial parts of Mussaenda recurvata. RSC Adv. 2023; 13(8): 5324-5336. doi:10.1039/D2RA06870B.
21. Berger M, Knittl-Frank C, Bauer S, Winter G and Maulide N. Application of relay C−H oxidation logic to polyhydroxylated oleanane triterpenoids. Chem. 2020; 6(5): 1183-1189. doi:10.1016/j.chempr.2020.04.007.
22. Renwick JD, Scopes PM and Huneck S. Optical rotatory dispersion and circular dichroism. Part LXIII. Unsaturated triterpene 28-carboxylic acids and related compounds. J Chem Soc C: Org. 1969; 1969(19): 2544-2549. doi:10.1039/J39690002544.
23. Bisht A, Avinash D, Sahu K K, Patel P, Das Gupta G and Kurmi B D, A comprehensive review on doxorubicin: mechanisms, toxicity, clinical trials, combination therapies and nanoformulations in breast cancer. Drug Deliv Transl Res. 2025; 15(1): 102-133. doi:10.1007/s13346-024-01648-0.
24. Kathleen G, Andrean G, Robert P and Bjoern-Oliver G. SuperPred 3.0: drug classification and target prediction—a machine learning approach. Nucleic Acids Res. 2022; 50(W1): W726-W731. doi: 10.1093/nar/gkac297.