Biosynthesis, Characterization, and Antihyperlipidemic Property of Green Silver Nanoparticles Derived from Borassus aethiopum Hypocotyl Extract in Poloxamer 407-Induced Hyperlipidemic Pre-Clinical Models
Main Article Content
Abstract
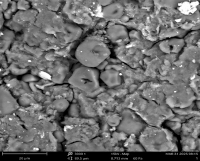
The use of silver nanoparticles (AgNPs) for hyperlipidemia is advancing nanocardiology research owing to their nano-size, targeted therapy, biomembrane penetrating ability and easy delivery. This study synthesized, characterized, and assessed the antihyperlipidemic potential of AgNPs derived from the aqueous extract of Borassus aethiopum hypocotyl (BAHAE) in poloxamer-407-induced hyperlipidemic rats. Thirty-five rats were divided into seven groups (n=5). Animals in Group A received distilled water (DW). Animals in Group B-G, which were induced into hyperlipidemia with poloxamer-407 (300 mg/kg BW), received DW, fenofibrate [standard medication] (250 mg/kg BW), 10 mg/kg BW nano-sized particles (NSP), 20 mg/kg BW NSP, 200 mg/kg BW of BAHAE, and 400 mg/kg BW of BAHAE, respectively. Treatment occurred for 14 days. Related bioassays and characterization were conducted using standard protocols. Poloxamer-407 which substantially (p<0.05) lowered HDLC, CAT, SOD, and GPx, significantly elevated serum levels of CK-MB, cTnI, LDH, MDA, TC, TAG, and LDLC. BAHAE and AgNPs restored the examined biomarkers, with profound effect from AgNPs than from BAHAE or fenofibrate. UV-Vis spectroscopy confirmed the synthesized AgNPs peak at 205 nm. FTIR indicated peaks/functional groups that are involved in the synthesis and stabilization of AgNPs, with a prominent peak at 991.5;79.4 wavelength/intensity. While XRD showed an amorphous/nanocrystalline structure with four intense peaks at 2θ angles of 19.01°, 31.10°, 37.50°, and 46.20°, SEM-EDX showed a rough and irregular surface with agglomerated particles. EDXRF spectrum showed the most prominent peak at ~22.16 keV. AgNPs normalized the poloxamer-407-induced hyperlipidemic alterations in rats and could be explored in the management of hyperlipidemia.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Visseren FLJ, Mach F, Smulders YM. ESC guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J. 2021; 42 (34): 3227–3237. https://doi.org/10.1093/eurheartj/ehab484.
2. Pirillo A, Norata GD. The burden of hypercholesterolemia and ischemic heart disease in an ageing world. Pharmacol Res. 2023; 193: 106814. https://doi.org/10.1016/j.phrs.2023.106814.
3. Pirillo A, Casula M, Olmastroni E, Norata GD, Catapano AL. Global epidemiology of dyslipidaemias. Nat Rev Cardiol. 2021; 18 (10): 689-700. doi: 10.1038/s41569-021- 00541 4.
4. WHO (World Health Organization), Global health observatory data repository, Geneva: World Health Organization; Available: https://apps.who.int/gho/data/view.main.2467?lang=en. 2016.
5. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. 2022; 118: 3272–3287.
6. Pirillo A, Tokgozoglu L, Catapano AL. European Lipid Guidelines and Cardiovascular Risk Estimation: Current Status and Future Challenges. Curr Atheroscler Rep. 2024; 26 (5): 133-137. doi: 10.1007/s11883-024-01194-7.
7. Oh RC, Trivette ET, Westerfield KL. Management of Hypertriglyceridemia: Common Questions and Answers. Am Family Phys. 2020; 102 (6): 347–354.
8. Thomson MJ, Serper M, Khungar V, Weiss LM, Trinh H, Firpi-Morell R, Roden M, Loomba R, Barritt AS, Gazis D, Mospan AR, Fried MW, Reddy KR, Lok AS. Prevalence and Factors Associated with Statin Use Among Patients with Nonalcoholic Fatty Liver Disease in the TARGET-NASH Study. Clin Gastroenterol Hepatol. 2022; 20 (2): 458-460.e4.
9. Sizar O, Khare S, Patel P, Talati R. Statin Medications. [Updated 2024 Feb 29]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing. Available from: https://www.ncbi.nlm.nih.gov/books/NBK430940/. 2024.
10. Egwu CO, Aloke C, Onwe KT, Umoke CI, Nwafor J, Eyo RA, Chukwu JA, Ufebe GO, Ladokun J, Audu DT, Agwu AO, Obasi DC, Okoro CO. Nanomaterials in Drug Delivery: Strengths and Opportunities in Medicine. Molecules. 2024; 29 (11): 2584. doi: 10.3390/molecules29112584.
11. Picraux ST. Nanotechnology. Encyclopedia Britannica, https://www.britannica.com/technology/nanotechnology. Accessed May 24, 2025.
12. Bayda S, Adeel M, Tuccinardi T, Cordani M, Rizzolio F. The History of Nanoscience and Nanotechnology: From Chemical-Physical Applications to Nanomedicine. Molecules. 2019; (25) (1): 72-112. doi: 10.3390/molecules25010112.
13. Vincent A, Esumaba SA, Jacob K, Nadratu MB, Fidelis MK. Development and Evaluation of African Palmyra Palm (Borassus aethiopum) Fruit Flour-Wheat Composite Flour Noodles. Congent Food Agric. 2020; 6 (1): 174-176.
14. Salako KV, Moreira F, Gbedomon RC, Tovissode F, Assogbadjo AE, Kakai RLG. Traditional Knowledge and Cultural Importance of Borassus aethiopum Mart. In Benin: Interacting Effects of Socio-Demographic Attributes and Multi-Scale Abundance. J Ethnobiol Ethnomed. 2018; 14(1): 36- 46.
15. Aduwamai UH, Mahmud BA, Daniel D. Antioxidant and Antihyperlipidemic Activity of Methanol Extract of Borassus aethiopum Fruit in Triton X-100 Induced Hyperlipidemic Rats. Am J Biochem. 2019; 9 (2): 35-44. doi: 10.5923/j.ajb.20190902.03.
16. Maniru N, Onuigwe FU, Wali U, Oluranti AC, Abdulrahman Y, Buhari HA, Kwaifa IK, Ibrahim AB. Effect of Methanolic Fruit Extract of Borassus aethiopum on Body Mass Index and Lipid Profile Parameters of High-Fat Diet-Induced Obese Wistar Rats. Bayero J Med Lab. Sci. 2024; 9 (1): 136–144.
17. Adams MD, Eze ED. Borassus aethiopum (Mart..) ethanol fruit extract reverses alloxan-treatment alterations in experimental animals, Mediterr. J Nutr Metab. 2022; 15 (3): 429-445. DOI:10.3233/MNM-211589
18. Naseem K, Zia-Ur-Rehman M, Ahmad A, Dubal D, Al-Garni TS. Plant Extract-Induced Biogenic Preparation of Silver Nanoparticles and Their Potential as Catalyst for the Degradation of Toxic Dyes. Coatings. 2020; 10(12):1235. https://doi.org/10.3390/coatings10121235
19. Li H, Dutkiewicz EP, Huang YC, Zhou HB, Hsu CC. Analytical methods for cholesterol quantification. J Food Drug Anal. 2019; 27 (2): 375-386. https://doi.org/10.1016/j.jfda.2018.09.001.
20. Warnick GR, Wood PD. National Cholesterol Education Program Recommendations for Measurement of High-Density Lipoprotein Cholesterol: Executive Summary, Clin Chem. 1995; 41 (10): 1427-1433.
21. Islam SMT, Osa-Andrews B, Jones PM, Muthukumar AR, Hashim I, Cao J. Methods of Low-Density Lipoprotein-Cholesterol Measurement: Analytical and Clinical Applications. EJIFCC. 2022; 33(4): 282-294.
22. Fossati P, Prencipe L. Serum triglycerides determined colorimetrically with an enzyme that produces hydrogen peroxide. Clin Chem. 1982; 28 (10): 2077–2080.
https://doi.org/10.1093/clinchem/28.10.2077.
23. Ben-Attig J, Latrous L, Galvan I, Zougagh M, Rios A. Rapid determination of malondialdehyde in serum samples using a porphyrin-functionalized magnetic graphene oxide electrochemical sensor. Anal Bioanal Chem. 2023; 415 (11): 2071-2080.
Doi: 10.1007/s00216-023-04594-x.
24. Krishna H, Avinash K, Shivakumar A, Al-Tayar NGS, Shrestha AK. A quantitative method for the detection and validation of catalase activity at physiological concentrations in human serum, plasma, and erythrocytes. (Spectrochimica Acta Part A). Mol Biomol Spectrosc. 2021; 251: 119358. https://doi.org/10.1016/j.saa.2020.119358.
25. Sun Y, Oberley L, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988; 34: 497–500. doi: 10.1093/clinchem/34.3.497.
26. Nwakulite A, Obeagu EI, Eze R, Ugochi VE, Vincent C, Okafor CJ, Chukwurah EF, Unaeze BC, Amaechi CO, Okwuanaso CB, Chukwuani U, Ifionu BI. Estimation of Serum Glutathione Peroxidase in Streptozotocin-Induced Diabetic Rat Treated with Bitter Leaf Extract. J Pharm Res Int’l. 2021; 33 (30B): 200–206. doi: 10.9734/jpri/2021/v33i30B31655.
27. Babuin L, Jaffe AS. Troponin: the biomarker of choice for the detection of cardiac injury. Can. Med Assoc J. 2005; 173 (10): 1191-1202. DOI: 10.1503/cmaj/051291.
28. Tate JR, Bunk DM, Christenson RH, Katrukha A, Noble JE, Porter RA, Schimmel H, Wang L, Panteghini M. Standardization of cardiac troponin I measurement: past and present. Pathol. 2010; 42 (5): 402-408. https://doi.org/10.3109/00313025.2010.495246.
29. Gerhardt W, Ljungdahl L, Borjesson J, Hofvendahl S, Hedenas B. Creatine kinase B-B-subunit activity in human serum. I. Development of an immunoinhibition method for routine determination of S-creatine kinase B-subunit activity. Clinica Chimica Acta. 1977; 78 (1): 29-41. https://doi.org/10.1016/0009-8981(77)90335-7.
30. Rchid SM, El-Azzouzi MK, Krimi K, El-Moujtahide D, Sebbar E, Choukri M.Verification of analytical performance of Creatine Kinase isoenzyme MB (CK-MB) on the Alinity c® Experience from the Biochemistry Laboratory of Mohammed VI University Hospital in Oujda. GSC Biol Pharm Sci. 2025; 30 (01): 201-205.
DOI: https://doi.org/10.30574/gscbps.2025.30.1.0026.
31. Glowacka J, Wisniewska A, Koncki R, Strzelak K. Photometric flow system for the determination of serum lactate dehydrogenase activity. Talanta. 2023; 265: 124817.
32. Sari D, Endardjo S, Irawati D. Blood lactate level in Wistar rats after four-and-twelve week intermittent aerobic training. Med J Indones. 2013; 22: 141-149. DOI: 10.13181/mji.v22i3.582].
33. Mallikarjuna RB, Vedavijaya T, Ramani YR, Sayana SB. Protective Role of Methanol Leaf Extract of Catharanthus Roseus in Lipid Profile Modulation in Diabetic Wistar Rats. Cureus. 2025; 14:17 (1): e77420. Doi: 10.7759/cureus.77420.
34. Atsukwei D, Eze ED, Adams MD, Seriki SA, Ukpabi CN. Hypolipidaemic Effect of Ethanol Leaf Extract of Moringa Oleifera Lam. in Experimentally induced Hypercholesterolemic Wistar Rats. Int’l J Nutr Food Sci. 2014; 3 (4): 355-360.
doi: 10.11648/j.ijnfs.20140304.28.
35. Ayo VI, Adondua MA, Morayo AE, Ekele J, Amilo D, Ochuele DA, Ayantse LM, Barrah C, Abdulsalam IO, Eya SB, Iheanacho CC, Tibile ST, Mohammed RI, Barde CE. Effect of Lactuca sativa supplemented diet on Poloxamer-407-induced hyperlipidemic albino rats (Rattus norvegicus). Asian J Nat Prod Biochem. 2023; 21 (2): 67-78. DOI: 10.13057/biofar/f210203.
36. Adams MD, Sharubutu BG, Olaolu TD. Molecular docking, HPLC phytochemical profiling and androgenic property of ethylacetate fraction of Borassus aethiopum (Mart.) hypocotyl in pre-clinical models. J Ethnopharmacol. 2026; 354: 120494. https://doi.org/10.1016/j.jep.2025.120494.
37. Adams MD, Manu HA, Enyioma-Alozie S. Molecular Docking, Contraceptive Property and Histopathological Changes in Experimental Models by Digitaria exilis Grain Extract via Interference with Steroidogenesis at Ovarian Level. Trop J Nat Prod Res. 2025; 9(7): 3349–3359. https://doi.org/10.26538/tjnpr/v9i7.6
38. Dzhelebov SP, Trifonova K. Poloxamer 407-induced chronic hyperlipidemia is not associated with disorders of blood glucose level. Trakia J Sci. 2025; 23 (1): 22-29.
doi:10.15547/tjs.2025.01.002.
39. Pham VAT, Pham PX, Tran TT, Dang HTT, Trinh QV, Dau DT. Effect of a nature-derived combination of Ananas comosus and Bambusa bambos (L.) in hyperlipidemic experimental animals. Trop J Nat Prod Res. 2025; 9 (5): 2323–2328.
https://doi.org/10.26538/tjnpr/v9i5.61.
40. Bawa I, Uti DE, Itodo MO, Umoru GU, Zakari S, Obeten UN. Effect of Solvent Extracts of Tephrosia vogelii Leaves and Stem on Lipid Profile of Poloxamer 407-Induced Hyperlipidemic Rats. Ibnosina J Med Biomed Sci. 2022; 14: 135–144. https://doi.org/ 10.1055/s-0042-1760223.
41. Naik HG, Kolur A, Maled D, Khanwelkar CC, Desai R, Gidamudi S. Effect of Poloxamer-407 on VLDL, LDL, and HDL. Nat J Physiol Pharm Pharmacol. 2014; 4 (3): 221–224. DOI: 10.5455/njppp.2014.4.040620141
42. Kelle BP, Cesic AK, Custovic S, Cosovic E, Lagumdzija D, Jordamovic N, Kusturica J. Improvement of a diet-induced model of hyperlipidemia in Wistar rats: Assessment of biochemical parameters, the thickness of the abdominal aorta, and liver histology. J King Saud Univ – Sci. 2024; 36 (2): 103068.
https://doi.org/10.1016/j.jksus.2023.103068.
43. Okere OS, Adams MD, Orji CG. Chemical Composition, In vivo Immunomodulatory and Anti-Hyperlipidaemic Properties of Rhinoceros (Rhino) Oil in Lead-Induced Immunocompromised Models. J Phytomed Therapeut. 2022; 21 (2): 931–974. https://dx.doi.org/10.4314/jopat.v21i2.15.
44. Nair SM, Pareek A, Jamali MC. Assessment of Biochemical Markers for Early Detection and Monitoring of Cardiovascular Diseases: Myocardial Infarction and Heart Failure. Qual Assur. 2024; 15 (1): 288-295. https://doi.org/10.25258/ijpqa.15.1.43.
45. Wu J, Qin M, Gao Y, Liu Y, Liu X, Jiang Y, Yang Y, Gao Y. Association between fluoride exposure and the risk of serum CK and CK-MB elevation in adults: a cross- sectional study in China. Front. Public Health. 2025; 12: 1410056. doi: 10.3389/fpubh.2024.1410056.
46. Herman E, Knapton A, Rosen E, Zhang J, Estis J, Agee SJ, Lu QA, Todd JA, Lipshultz SE. Baseline serum cardiac troponin I concentrations in Sprague-Dawley, spontaneous hypertensive, Wistar, Wistar-Kyoto, and Fisher rats as determined with an ultrasensitive immunoassay, Toxicol Pathol. 2011; 39 (4): 653-663. doi: 10.1177/0192623311406931.
47. Reuben E, Chinko BC, Batubo NP, Amah-Tariah FS. Impact of Intermittent Fasting on Serum Cardiac Biomarkers in Male Wistar Rats. Int’l J Clin Exp Med Sci. 2025; 11(1): 11-17. https://doi.org/10.11648/j.ijcems.20251101.12.
48. Rahman MF, Siddiqui, MK, Jamil K. LDH profiles of male and female rats treated with Vepacide. Phytother Res. 2002; 16(2): 122-126. doi: 10.1002/ptr.809.
49. Guo P, Ding H, Li X, Xie D, Wang K, Su W, Yang X, Nie F, Wang P. Association between lactate dehydrogenase levels and all-cause mortality in ICU patients with heart failure: a retrospective analysis of the MIMIC-IV database, BMC Cardiovasc Disord. 2025; 25 (62): 121- 129. https://doi.org/10.1186/s12872-025-04513-1.
50. van Deel ED, Lu Z, Xu X, Zhu G, Hu XT, Oury D, Bache RJ, Duncker DJ, Chen Y.
Extracellular superoxide dismutase protects the heart against oxidative stress and hypertrophy after myocardial infarction. Free Radic Biol Med. 2008; 1:44 (7): 1305-1313.
Doi: 10.1016/j.freeradbiomed.2007.12.007.
51. Cairns M, Odendaal C, O’Brien C, Marais E, Oestlund I, Storbeck K, Sishi B, Joseph D, Smith C, Essop MF. Effects of chronic stress on rat heart function following regional ischemia: a sex-dependent investigation. Am J Physiol-Heart Circulat Physiol. 2024; 327 (4): H880-H895. https://doi.org/10.1152/ajpheart.00424.2024.
52. Zhao B, Peng J, Chen C, Fan Y, Zhang K, Zhang Y, Huang X. Innovative engineering of superoxide dismutase for enhanced cardioprotective biocatalysis in myocardial ischemia-reperfusion injury. Int’l J Biol Macromol. 2025; 286: 137656. https://doi.org/10.1016/j.ijbiomac.2024.137656.
53. Qin F, Lennon-Edwards S, Lancel S, Biolo A, Siwik DA, Pimentel DR, Dorn GW, Kang YJ, Colucci WS. Cardiac-specific overexpression of catalase identifies hydrogen peroxide-dependent and -independent phases of myocardial remodeling and prevents the progression to overt heart failure in G(alpha)q-overexpressing transgenic mice. Circ Heart Fail. 2010; 3 (2): 306-313.
Doi: 10.1161/CIRCHEARTFAILURE.109.864785.
54. Pendergrass KD, Varghese ST, Maiellaro-Rafferty K, Brown ME, Taylor WR. Davis ME, Temporal Effects of Catalase Overexpression on Healing After Myocardial Infarction, Circ: Heart Fail. 2011; 4 (1): 98-106.
https://doi.org/10.1161/CIRCHEARTFAILURE.110.957712.
55. Lankin VZ, Tikhaze AK, Melkumyants AM. Malondialdehyde as an Important Key Factor of Molecular Mechanisms of Vascular Wall Damage under Heart Disease Development. Int’l J Mol Sci. 2023; 24 (1): 128. https://doi.org/10.3390/ijms24010128.
56. Hadi AN, Zaidi IA, Kamal Z, Ashraf AD, Khan RU, Rumman GT, Khan MH, Omair F. Estimation of Serum Malondialdehyde (a Marker of Oxidative Stress) as a Predictive Biomarker for the Severity of Coronary Artery Disease (CAD) and Cardiovascular Outcomes, Cureus. 2024; 16 (9): e69756. DOI 10.7759/cureus.69756.
57. Ojetola AA, Adedeji TG, Fasanmade AA. Changes in antioxidant status, atherogenic index, and cardiovascular variables after prolonged doses of D-ribose-L-cysteine in male Wistar rats. Heliyon. 2021; 7 (2): e06287. https://doi.org/10.1016/j.heliyon.2021.e06287.
58. Eze ED, Afodun AM, Sulaiman SO, Ponsiano N, Iliya E, Adams MD, Okpanachi AO, Rabiu KM. Lycopene attenuates diabetes-induced oxidative stress in Wistar rats. J Diab Endocrinol. 2018; 9 (2): 11-19. DOI: 10.5897/JDE2018.0118.
59. Abdel-Hafeez AM, Abdel-Goad MA. Silver Nanoparticles from Nature: Green Synthesis
Methods and Applications - A Review. J Adv Engr Trend. 2025; 41 (4): 227-236.
60. Toufique A, Tugrul RO, Gulnaz O. Multifarious Uses of UV-VIS Spectroscopy for Green Synthesis of Silver Nanoparticles for Antibacterial Textiles. Textil Leath Rev. 2024; 7: 176-202. https://doi.org/10.31881/TLR.2024.014.
61. Villagran Z, Anaya-Esparza LM, Velazquez-Carriles CA, Silva-Jara JM, Ruvalcaba-Gomez JM, Aurora-Vigo EF. Rodriguez-Lafitte E, Rodriguez-Barajas N, Balderas-Leon I, Martinez-Esquivias F, Plant-Based Extracts as Reducing, Capping, and Stabilizing Agents for the Green Synthesis of Inorganic Nanoparticles. Resour. 2024; 13 (6): 70. https://doi.org/10.3390/resources13060070.
62. Pasieczna-Patkowska S, Cichy M, Flieger J. Application of Fourier Transform Infrared (FTIR) Spectroscopy in Characterization of Green Synthesized Nanoparticles. Molecules. 2025; 30 (3): 684. https://doi.org/10.3390/molecules30030684.
63. Lekkala VD, Muktinutalapati VVAV, Lebaka VR, Lomada D, Korivi M, Li W, Reddy MC. Green Synthesis and Characterization of Silver Nanoparticles from Tinospora cordifolia Leaf Extract: Evaluation of Their Antioxidant, Anti-Inflammatory, Antibacterial, and Antibiofilm Efficacies. Nanomaterials. 2025; 15 (5): 381-389. https://doi.org/10.3390/nano15050381.
64. Kakol M, Tagliasacchi E, Borkowski A, Slowakiewicz M. Influence of different sample preparation techniques on imaging viruses and virus-like particles by scanning electroand scanning transmission electron microscopes. Front. Microbiol. 2023; 14: 1279720. doi: 10.3389/fmicb.2023.12797
65. Irshad M, Shah L, Shujaat N, Khan M, Niaz Z, Ullah I. Green Synthesis and Characterization of Silver Nanoparticles Using Lespedeza juncea Extract: An Insight Into Its Antibacterial, Antifungal, and Enzyme Inhibitory Potential. Microsc Res Tech. 2025; 16: 1-15. doi: 10.22541/au.172892530.01193795/v1.
66. El-Baz YG, Moustafa A, Ali MA, El-Desoky GE, Wabaidur SM, Iqbal A. Green synthesized silver nanoparticles for the treatment of diabetes and the related complications of hyperlipidemia and oxidative stress in diabetic rats. Exp Biol Med. 2024; 248 (23): 2237-2248. doi:10.1177/15353702231214258
67. Saba M, Farooq S, Alessa AH, Bektas KI, Belduz AO, Khan AZ, Shah AA, Badshah M, Khan S. Green synthesis of silver nanoparticles using Keratinase from Pseudomonas aeruginosa-C1M, characterization and applications as novel multifunctional biocatalyst. BMC Biotechnol. 2025; 25: 27. https://doi.org/10.1186/s12896-025-00959-5.
68. Shitu IG, Katibi KK, Taura LS, Muhammad A, Chiromawa IM, Adamu SB, Iya SGD. X- ray diffraction (XRD) profile analysis and optical properties of Klockmannite copper selenide nanoparticles synthesized via microwave-assisted technique. Ceramics International. 2023; 49(8): 12309-12326. https://doi.org/10.1016/j.ceramint.2022.12.086.
69. Abbas R, Luo J, Qi X, Naz XA, Khan IA, Liu H, Yu S, Wei J. Silver Nanoparticles: Synthesis, Structure, Properties and Applications. Nanomaterials. 2024; 14 (17): 1425. https://doi.org/10.3390/nano14171425.
70. Dekeyrel J, Atkinson R, Chavez E, da-Silva M, Castano OI, Pulleman M, Smolders E. Using optimized monochromatic energy dispersive X-ray fluorescence to determine the cadmium concentration in cacao and soil samples. Heliyon. 2024; 10(20): e39034, https://doi.org/10.1016/j.heliyon.2024.e39034.
71. Karuppannan P, Saravanan K, Egbuna C, Uche CZ, Patrick-Iwuanyanwu KC, Khan J. Antihyperlipidemic Effects of Silver Nanoparticles Synthesized from Ventilago maderaspatana Leaf Extract on Streptozotocin-Induced Albino Rats. Trop J Nat Prod Res. 2021; 5 (6): 1066-1071. doi.org/10.26538/tjnpr/v5i6.14.
72. Siddique NA, AL-Samman AM, Kahkashan MA. GC–MS analysis and green synthesis of silver nanoparticles using Emblica officinalis leaves and evaluation of its antioxidant and in-vitro anti-hypercholesterolemic potential. J Taibah Univ Sci. 2024; 18 (1): 2390211. https://doi.org/10.1080/16583655.2024.2390211.