The Effect of Bovine Femur and Rib Bone Extract on α-Amylase Enzyme and Blood Sugar Levels in Streptozotocin (STZ)-Induced Diabetic Rats
Main Article Content
Abstract
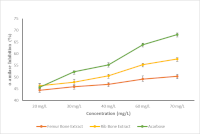
Diabetes mellitus is a non-communicable disease that remains a global health problem with increasing prevalence, and the search for natural sources that may help regulate blood glucose levels is ongoing. Therefore, this study aimed to develop a protein-rich extract from bovine femur and rib bones prepared by the boiling technique for 24 hours, and evaluate its effects on fasting blood glucose (FBG) in diabetic rats and its inhibitory activity against α-amylase. Twenty-eight Wistar rats were divided into seven groups: Normal group (NG): no streptozotocin (STZ) induction; Negative control group (NC): induced with STZ (50 mg/kg) without treatment; Positive control group (PC): STZ induction + acarbose (0.9 mg); Treatment groups: STZ induction + bone extracts at 500 mg/kg (R1 and F1) and 1000 mg/kg (F2 and R2) orally, once daily for 30 days. FBG were assessed before induction (day 0), after induction (day 6), and 30 days post-treatment. The results showed that FTIR analysis revealed a characteristic amide absorption consistent with proteins and peptides, and the extracts demonstrated α-amylase inhibitory activity with IC₅₀ of 37.41 and 66.87 mg/L for rib and femur extract, respectively. Administration of bone extracts decreased FBG in both groups (F and R) compared with the control groups (NC and PC), although the differences were not statistically significant (p>0.05). These results indicate that bone extracts have the potential to be used as an adjunctive therapy for diabetes management.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.International Diabetes Federation. IDF Diabetes Atlas 10th edition. 2021. Available from: https://diabetesatlas.org/data/en/
2.Indonesian Endocrinology Association. Guidelines for the Management and Prevention of Type 2 Diabetes Mellitus in Indonesia. Jakarta: PB PERKENI. 2021.
3.Health Development Policy Agency. Indonesian Health Survey. Jakarta: Ministry of Health of Indonesia. 2023. 232-254 p.
4.Roden M and Shulman GI. The Integrative Biology of Type 2 Diabetes. Nature. 2019; 576(7785):51-60. doi: 10.1038/s41586-019-1797-8.
5.Gidado A, Watafua M, Tagi HS, Sa’ad RS, Abdullahi S. Alpha-Amylase, Maltase and Sucrase Inhibitions by Aqueous Leaf Extracts of Anacardium occidentale (Anacardiacea) and Piliostigma reticulatum (Caesalpiniaceae) in Rats. Trop J Nat Prod Res. 2019; 3(6):210-215.
6.Fadimu GJ, Farahnaky A, Gill H, Olalere OA, Gan CY, Truong T. In-Silico Analysis and Antidiabetic Effect of α-Amylase and α-Glucosidase Inhibitory Peptides from Lupin Protein Hydrolysate: Enzyme-Peptide Interaction Study Using Molecular Docking Approach. Foods. 2022; 11(21):3375.
7.Alqahtani AS, Hidayathulla S, Rehman MT, ElGamal AA, Al-Massarani S, Razmovski-Naumovski V. Alpha-Amylase and Alpha-Glucosidase Enzyme Inhibition and Antioxidant Potential of 3-Oxolupenal and Katononic Acid Isolated from Nuxia oppositifolia. Biomolecules. 2019; 10(1):61.
8.Dewi I, Chodidjah C, Atina H. Evaluation of Clitoria ternatea L. Flower Extract in Preventing Complications of Diabetes Mellitus. Trop J Nat Prod Res. 2023; 7(10):4908-4911.
9.Gómez-Guillén MC, Giménez B, López-Caballero ME, Montero MP. Functional and Bioactive Properties of Collagen and Gelatin from Alternative Sources: A Review. Food Hydrocolloids. 2011; 25(8):1813–1827.
10.Nong NTP and Hsu JL. Characteristics of Food Protein-Derived Antidiabetic Bioactive Peptides: A Literature Update. Int J Mol Sci. 2021; 22(17):9508.
11.Ma C, Tian X, Li Y, Guo J, Wang X, Chen S. Using High-Temperature Cooking for Different Times for Bone Soup: Physicochemical Properties, Protein Oxidation and Nutritional Value. J Food Comp Anal. 2023; 122:105467.
12.Sulochana K, Srinivasan V, Radhakrishnan S, Angayarkanni N. Antidiabetic Effect of Free Amino Acids Supplementation in Human Visceral Adipocytes Through Adiponectin-Dependent Mechanism. Indian J Med Res. 2019; 149(1):41-46.
13.Kim HJ, Kim D, Chae HS, Kim NY, Jang A. Nutritional Composition in Bone Extracts from Jeju Crossbred Horses at Different Slaughter Ages. Korean J Food Sci Anim Resour. 2017; 37(4):486–493.
14.Hsu D, Lee C, Tsai W, Chien Y. Essential and Toxic Metals in Animal Bone Broths. Food Nutr Res. 2017; 61(1):1347478.
15.Algehainy NA, Mohamed EM, Aly HF, Younis EA, Altemani FH, Alanazi MA. Nutritional Composition and Anti-Type 2 Diabetes Mellitus Potential of Femur Bone Extracts from Bovine, Chicken, Sheep, and Goat: Phytochemical and In Vivo Studies. Nutrients. 2023; 15(18):4037.
16.Seol JY, Yoon JY, Jeong HS, Joo N, Choi SY. Anti-Aging Effects of the Hanwoo Leg Bone, Foot and Tail Infusions (HLI, HFI and HTI) on Skin Fibroblast. Korean J Food Sci Anim Resour. 2016; 36(2):237–243.
17.Mar-Solís LM, Soto-Domínguez A, Rodríguez-Tovar LE, Rodríguez-Rocha H, García-García A, Aguirre-Arzola VE. Analysis of the Anti-Inflammatory Capacity of Bone Broth in a Murine Model of Ulcerative Colitis. Medicina. 2021; 57(11):1138.
18.Choi HG, Choi HS, Choi YS, Jung MO, Choi JS, Choi YI. Effects of Mixed Bone and Brisket Meat on Physico-Chemical Characteristics of Shank Bone and Rib Extracts from Hanwoo. Korean J Food Sci Anim Resour. 2016; 36(1):61–67.
19.Kielkopf CL, Bauer W, Urbatsch IL. Bradford Assay for Determining Protein Concentration. Cold Spring Harb Protoc. 2020; 2020(4):102269.
20.Sandt C. Identification and Classification of Proteins by FTIR Microspectroscopy. Biochim Biophys Acta Gen Subj. 2024; 1868(10):130688.
21.Mogole L, Omwoyo W, Mtunzi F. Phytochemical Screening, Anti-Oxidant Activity and α-Amylase Inhibition Study Using Different Extracts of Loquat (Eriobotrya japonica) Leaves. Heliyon. 2020; 6(8):e04736.
22.Federer WT. Randomization and Sample Size in Experimentation. 1966. Available from: https://ecommons.cornell.edu/
23.Institutional Animal Care and Use Committee Guidebook. 2002. Available from: https://grants.nih.gov/grants/olaw/guidebook.pdf
24.McIver LA, Preuss CV, Tripp J. Acarbose. [Updated 2024 Feb 12]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493214/
25.Food and Drug Regulatory Agency of Indonesia. Guidelines for Preclinical Pharmacodynamic Testing of Traditional Medicines. Jakarta: Percetakan Negara. 2023.
26.Fajarwati I, Solihin DD, Wresdiyati T, Batubara I. Self-Recovery in Diabetic Sprague Dawley Rats Induced by Intraperitoneal Alloxan and Streptozotocin. J Exp Biol. 2023; 9(5):e15533.
27.Shaker G and Zubair M. Peroxidase-Coupled Glucose Method. 2025. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025. PMID: 37603668.
28.Brady PN and Macnaughtan MA. Evaluation of Colorimetric Assays for Analyzing Reductively Methylated Proteins:
Biases and Mechanistic Insights. Anal Biochem. 2015; 491(1):43–51.
29.Amirrah IN, Lokanathan Y, Zulkiflee I, Wee MFMR, Motta A, Fauzi MB. A Comprehensive Review on Collagen Type I Development of Biomaterials for Tissue Engineering: From Biosynthesis to Bioscaffold. Biomedicines. 2022; 10(9):2307.
30.Losso JN and Ogawa M. Thermal Stability of Chicken Keel Bone Collagen. J Food Biochem. 2013; 38(3):345–351.
31.Chimegee N and Dashmaa D. The Daily Value of Micronutrients in Newly Produced Beef and Horse Concentrated Bone Broths. Mong J Agric Sci. 2018; 23(01):30–34.
32.McMurry J. Fundamentals of Organic Chemistry. 7th ed. California: Mary Finch. 2011. 438-440 p.
33.Nandiyanto ABD, Oktiani R, Ragadhita R. How to Read and Interpret FTIR Spectroscope of Organic Material. Indones J Sci Technol. 2019; 4(1):97–118.
34.Yang H, Yang S, Kong J, Dong A, Yu S. Obtaining Information About Protein Secondary Structures in Aqueous Solution Using Fourier Transform IR Spectroscopy. Nat Protoc. 2015; 10(3):382–396.
35.de Campos Vidal B and Mello MLS. Collagen Type I Amide I Band Infrared Spectroscopy. Micron. 2011; 42(3):283–289.
36.Chatterley AS, Laity P, Holland C, Weidner T, Woutersen S, Giubertoni G. Broadband Multidimensional Spectroscopy Identifies the Amide II Vibrations in Silkworm Films. Molecules. 2022; 27(19):6275.
37.Wang Z, Zhang Y, Zhang J, Huang L, Liu J, Li Y. Exploring Natural Silk Protein Sericin for Regenerative Medicine: an Injectable, Photoluminescent, Cell-Adhesive 3D Hydrogel. Sci Rep. 2014; 4:6825.
38.Kashtoh H and Baek KH. New Insights into the Latest Advancement in α-Amylase Inhibitors of Plant Origin with Anti-Diabetic Effects. Plants. 2023; 12(16):2944.
39.Bashary R, Vyas M, Nayak SK, Suttee A, Verma S, Narang R. An Insight of Alpha-amylase Inhibitors as a Valuable Tool in the Management of Type 2 Diabetes Mellitus. Curr Diab Rev. 2020; 16(2):117–136.
40.Ononamadu C, Ezeigwe O, Owolarafe T, Ihegboro G, Lawal T, Salawu K. Starch-Iodine Assay Method Underestimates α-Amylase Inhibitory Potential of Antioxidative Compounds and Extracts. BioTechnologia. 2020; 101(1):45–54.
41.Aleixandre A, Gil JV, Sineiro J, Rosell CM. Understanding Phenolic Acids Inhibition of α-Amylase and α-Glucosidase and Influence of Reaction Conditions. Food Chem. 2022; 372:131231.
42.Oztug M. Bioactive Peptide Profiling in Collagen Hydrolysates: Comparative Analysis Using Targeted and Untargeted Liquid Chromatography–Tandem Mass Spectrometry Quantification. Molecules. 2024; 29(11):2592.
43.Rico-Llanos GA, Borrego-González S, Moncayo-Donoso M, Becerra J, Visser R. Collagen Type I Biomaterials as Scaffolds for Bone Tissue Engineering. Polymers. 2021; 13(4):599.
44.Yoon HJ, Kim SB, Somaiya D, Noh MJ, Choi KB, Lim CL. Type II Collagen and Glycosaminoglycan Expression Induction in Primary Human Chondrocyte by TGF-β1. BMC Musculoskelet Disord. 2015; 16(1):144.
45.Mariné-Casadó R, Domenech-Coca C, Fernández S, Costa A, Segarra S, López-Andreo MJ. Effects of the Oral Administration of Glycosaminoglycans With or Without Native Type II Collagen on the Articular Cartilage Transcriptome in an Osteoarthritic-Induced Rabbit Model. Genes Nutr. 2024; 19(1):49.
46.Gilbert ER, Fu Z, Liu D. Development of a Nongenetic Mouse Model of Type 2 Diabetes. Exp Diabetes Res. 2011; 2011:652514.
47.Nahdi AMTA, John A, Raza H. Elucidation of Molecular Mechanisms of Streptozotocin-Induced Oxidative Stress, Apoptosis, and Mitochondrial Dysfunction in Rin-5F Pancreatic β-Cells. Oxid Med Cell Longev. 2017; 2017:3465928.
48.Titisari N, Ahmad H, Samsulrizal N, Fauzi A, Razak I. The Mechanism Underlying Streptozotocin Injection for the Development of a Nontransgenic Alzheimer’s Disease Animal Model. Open Vet J. 2025; 15(2):594-600.
49.Deeds MC, Anderson JM, Armstrong AS, Gastineau DA, Hiddinga HJ, Jahangir A. Single Dose Streptozotocin-Induced Diabetes: Considerations for Study Design in Islet Transplantation Models. Lab Anim. 2011; 45(3):131–140.
50.Das RR, Keshri USP, Das N, Das G. Streptozotocin (Streptozocin: STZ) as a Diabetic Agent: A Narrative Review and Update. Int J Toxicol Pharmacol Res. 2023; 13(4):172–177.
51.van Gerwen J, Shun-Shion AS, Fazakerley DJ. Insulin Signalling and GLUT4 Trafficking in Insulin Resistance. Biochem Soc Trans. 2023; 51(3):1057–1069.
52.Li M, Chi X, Wang Y, Setrerrahmane S, Xie W, Xu H. Trends in Insulin Resistance: Insights into Mechanisms and Therapeutic Strategy. Signal Transduct Target Ther. 2022; 7(1):142.
53.Ghasemi A and Jeddi S. Streptozotocin as a Tool for Induction of Rat Models of Diabetes: a Practical Guide. Excli J. 2023; 22:274–294.
54.Nir T, Melton DA, Dor Y. Recovery from Diabetes in Mice by β Cell Regeneration. J Clin Invest. 2007; 117(9):2553–2561.
55.Wang L, Wang Q, Qian J, Liang Q, Wang Z, Xu J. Bioavailability and Bioavailable Forms of Collagen After Oral Administration to Rats. J Agric Food Chem. 2015; 63(14):3752–3756.
56.Geahchan S, Baharlouei P, Rahman A. Marine Collagen: A Promising Biomaterial for Wound Healing, Skin Anti-Aging, and Bone Regeneration. Mar Drugs. 2022; 20(1):61.