Melastoma malabathricum and Muntingia Calabura against Colon Cancer Cell Line and DMH-Induced Rat Model: In Vitro and In Vivo Perspective
Main Article Content
Abstract
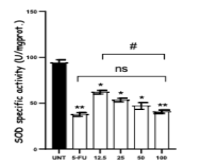
Melastoma malabathricum (MM) and Muntingia calabura (MC) are traditionally used medicinal plants. The present study examined the anti-colon cancer effects of a water-based extract derived from the combined leaves of both plants (MM-MC). Cell lethality was tested in HCT116 human colon cancer cells, where MM-MC induced cell shrinkage. The impact of the combined extract on apoptosis induction, cell cycle regulation, and oxidative stress was analyzed in HCT116 cells. The extract amplified the expression of Bax and caspase-3, while repressing Bcl-2 and Cyclin B1. Additionally, MM-MC suppressed the activities of antioxidant enzymes (CAT, GSH-Px, SOD) and the total antioxidant capacity (T-AOC), while it increased MDA and total oxidant status (TOS) levels in HCT116 cells. In the 1,2-dimethylhydrazine (DMH)-exposed rats, oral administration of MM-MC at 250 and 500 mg/kg markedly inhibited aberrant crypt foci (ACF) formation, achieving 71.42% and 85.68% inhibition, respectively. The extract also restored normal colonic architecture and decreased the proportion of PCNA-positive nuclei in the distal colon to 11% and 7% in the 250 and 500 mg/kg treatment groups, as opposed to 16% observed in the untreated DMH-induced control group. These findings demonstrate the anti-colorectal cancer potential of the MM-MC extract, highlighting its potential use as a treatment for colon cancer.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics. GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021; 71(3):209–249.
2.Morgan E, Arnold M, Gini A, Lorenzoni V, Cabasag CJ, Laversanne M, Vignat J, Ferlay J, Murphy N, Bray F. Global Burden of Colorectal Cancer in 2020 and 2040: Incidence and Mortality Estimates from GLOBOCAN. Gut. 2023; 72(2):338-344.
3.Bhargavi R, Shreyas VD. Colorectal Cancer: Etiology, Pathogenesis and Current Treatment. J Innov Pharm Biol Sci. 2020; 7(4):20-24.
4.Wang H, Khor TO, Shu L, Su ZY, Fuentes F, Lee JH, Kong AN. Plants vs. Cancer: A Review on Natural Phytochemicals in Preventing and Treating Cancers and their Druggability. Anticancer Agents Med Chem. 2012; 12(10):1281-1305.
5.Dzobo K. The Role of Natural Products as Sources of Therapeutic Agents for Innovative Drug Discovery. Compr Pharmacol. 2022; 2:408–422.
6.Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell. 2011; 144(5):646-674.
7.Seril DN, Liao J, Yang GY, Yang CS. Oxidative Stress and Ulcerative Colitis-Associated Carcinogenesis: Studies in Humans and Animal Models. Carcinogenesis. 2003; 24(3):353-362.
8.Nelson VK, Nuli MV, Mastanaiah J, Saleem TSM, Birudala G, Jamous YF, Alshargi O, Kotha KK, Sudhan HH, Mani RR, Muthumanickam A, Niranjan D, Jain NK, Agrawal A, Jadon AS, Mayasa V, Jha NK, Kolesarova A, Slama P, Roychoudhury S. Reactive Oxygen Species Mediated Apoptotic Death of Colon Cancer Cells: Therapeutic Potential of Plant Derived Alkaloids. Front Endocrinol. 2023; 14:1201198
9.El-Seedi HR, El-Said AMA, Khalifa SAM, Göransson U, Bohlin L, Borg-Karlson AK, Verpoorte R. Biosynthesis, Natural Sources, Dietary Intake, Pharmacokinetic Properties and Biological Activities of Hydroxycinnamic Acids. J Agric Food Chem. 2012; 60(44):10877–10895.
10.Zakaria ZA, Zainol ASN, Sahmat A, Salleh NI, Hizami A, Mahmood ND, Nasir N, Mamat SS, Kamisan FH, Mohtarrudin N, Abdul Hamid SS, Tohid SF, Teh LK, Salleh MZ. Gastroprotective Activity of Chloroform Extract of Muntingia calabura and Melastoma malabathricum Leaves. Pharm Biol. 2016; 54(5):812–826.
11.Lestari OA, Palupi NS, Setiyono A, Kusnandar F, Yuliana ND. In vitro Antioxidant Potential and Phytochemical Profiling of Melastoma malabathricum Leaf Water Extract. Food Sci Technol (Brazil). 2022; 42(1): e92021.
12.Kumar V, Sachan R, Rahman M, Rub RA, Patel DK, Sharma K, Gahtori P, Al-abbasi FA, Alhayyani S, Anwar F, Kim HS. Chemopreventive Effects of Melastoma malabathricum L. Extract in Mammary Tumor Model via Inhibition of Oxidative Stress and Inflammatory Cytokines. Biomed Pharmacother. 2021; 137:111298.
13.Zakaria ZA, Kamsani NE, Azizah R, Sulistyorini L. Anticarcinogenic Activity of Methanol Extract of Melastoma malabathricum Leaves is Attributed to the Presence of Phenolics Compounds and the Activation of Endogenous Antioxidant System. Bol Latinoam Caribe Plant Med Arom. 2022; 21(1):66–80.
14.Kamsani NE, Zakaria ZA, Md Nasir NL, Mohtarrudin N, Mohamad ANB. Safety Assessment of Methanol Extract of Melastoma malabathricum L. Leaves Following the Subacute and Subchronic Oral Consumptions in Rats and Its Cytotoxic Effect Against the HT29 Cancer Cell Line. Evid-Based Complement Alternat Med. 2019; 4:1-4.
15.Nasir Md NL, Kamsani NE, Mohtarrudin N, Md Tohid, SF, Zakaria ZA. Safety Evaluation of Orally-Administered Methanol Extract of Muntingia Calabura Linn. Leaves: A Sub-Chronic Toxicity Study in Sprague Dawley Rats. Pak J Pharm Sci. 2020; 33(5):2009–2016.
16.Zakaria N, Mohd YN, Zakaria Z, Widera D, Yahaya BH. Inhibition of NF-κB Signaling Reduces the Stemness Characteristics of Lung Cancer Stem Cells. Front Oncol. 2018; 17(8):166.
17.Zakaria ZA, Nasir NLM, Kamsani NE, Khaza’ai H, Sulistyorini L, Azizah R. Ethyl Acetate Partition Obtained from The Methanol Extract of Muntingia Calabura Leaf Exerts Effective In vitro Antiproliferative Activity Against the HT-29 Colon Cancer. Bol Latinoam Caribe Plant Med Arom. 2022; 21(5):654–670.
18.Halim SZ, Zakaria ZA, Omar MH, Mohtarrudin N, Wahab IRA, Abdullah MNH. Synergistic Gastroprotective Activity of Methanolic Extract of a Mixture of Melastoma malabathricum and Muntingia calabura Leaves in Rats. BMC Compl Altern Med, 2017; 17(1):488.
19.Nelson VK, Sahoo NK, Sahu M, Sudhan HH, Pullaiah CP, Muralikrishna KS. In vitro anticancer activity of Eclipta alba whole plant extract on colon cancer cell HCT-116. BMC Complement Med Ther. 2020; 20(1):355.
20.Pham TL, He J, Kakazu AH, Calandria J, Do KV, Nshimiyimana R, Petasis NA, Bazan HEP, Bazan NG. Elovanoid-N32 or RvD6-Isomer Decrease ACE2 and Binding of S Protein RBD After Injury or INFγ in the Eye. Exp Eye Res. 2020; 199:108201.
21.Chen J, Kung-Wen L, Jau-Hong L, Chin-Chung Y and Jing-Gung C. Gypenosides Induced Apoptosis in Human Colon Cancer Cells through the Mitochondria-Dependent Pathways and Activation of Caspase-3. Anticancer Res. 2006; 26:(6B):4313-4326.
22.Sung B, Kang YJ, Kim DH, Hwang SY, Lee Y, Kim M, Yoon JH, Kim CM, Chung HY, Kim ND. Corosolic Acid Induces Apoptotic Cell Death in HCT116 Human Colon Cancer Cells through a Caspase-dependent pathway. Int J Mol Med. 2014; 33(4):943–949.
23.Razak S, Afsar T, Ullah A, Almajwal A, Alkholief M, Alshamsan A, Jahan S. Taxifolin, a Natural Flavonoid Interacts with Cell Cycle Regulators Causes Cell Cycle Arrest and Causes Tumor Regression by Activating Wnt/ Β -Catenin Signaling Pathway. BMC Cancer. 2018; 18(1):1043.
24.Alshammari GM, Balakrishnan A, Subash-Babu P, Al-khalifa A, Abdullah AA, ElGasim Ahmed YA, Naif AL, Hamed AG, Albekairi NA. Alpha-linolenic Acid Rich Allium porrum Methanolic Extract Potentially Inhibits HT-115 Human Colon Cancer Cells Proliferation via Mitochondria-mediated Apoptotic Mechanism. J King Saud Univ Sci. 2022; 34(1):101736.
25.Perillo B, di Donato, M, Pezone A, di Zazzo E, Giovannelli P, Galasso G, Castoria G, Migliaccio A. ROS in Cancer Therapy: The Bright Side of the Moon. Exp Mol Med. 2020; 52(2):192–203.
26.Pakpisutkul J, Suwapraphan J, Sripayak N, Sitkhuntod N, Loyrat S, Yahayo W, Supabphol R. The Effects of Vernonia cinerea Less Extracts on Antioxidant Gene Expression in Colorectal Cancer Cells. Asian Pac J Cancer Prev. 2022; 23(11):3923–3930.
27.Zhang Y, Guo Y, Wang M, Dong H, Zhang J, Zhang L. Quercetrin from Toona sinensis Leaves Induces Cell Cycle Arrest and Apoptosis Via Enhancement of Oxidative Stress in Human Colorectal Cancer SW620 Cells. Oncol Rep. 2017; 38(6)3319–3326.
28.Brenner SA, Zacheja S, Schäffer M, Feilhauer K, Bischoff SC, Lorentz A. Soluble CD14 Is Essential for Lipopolysaccharide-Dependent Activation of Human Intestinal Mast Cells from Macroscopically Normal as Well as Crohn's Disease Tissue. Immunol. 2024; 143(2):174–183.
29.Raskov H, Pommergaard HC, Burcharth J, Rosenberg J. Colorectal Carcinogenesis--Update and Perspectives. World J Gastroenterol. 2014; 20(48):18151-18164.
30.Mrudula K, Hemant U. Chemoprevention of DMH Induced Colon Carcinogenesis by Combination Administration of Lactobacillus acidophilus, Calcium and Moringa oleifera Leaves Extract. Int J Pharm Qual Assur. 2023; 14(4):1002-1010.
31.Sayed A, Youssef EA, Mahmoud SA, Youssry S, Abdel-Mawla AA. Effect of Niclosamide on Colorectal Cancer Induced by Dimethylhydrazine in Albino Mice. Egypt J Basic Appl Sci. 2023; 10(1):846–860.
32.Wang C, Qiao X, Wang J, Yang J, Yang C, Qiao Y, Guan Y, Wen A, Jiang L. Amelioration of DMH-induced colon cancer by eupafolin through the reprogramming of apoptosis-associated p53/Bcl2/Bax signaling in rats. Eur J Inflamm. 2022; 20: 1–15.
33.Yang Q, Ou C, Liu M, Xiao W, Wen C, Sun F. NRAGE Promotes Cell Proliferation by Stabilizing PCNA in a ubiquitin-proteasome Pathway in Esophageal Carcinomas. Carcinogenesis. 2014; 35:1643–1651.
34.Ban QJ, Ali HH, Hussein AG. (2012). Immunohistochemical Expression of PCNA and CD34 in Colorectal Adenomas and Carcinomas Using Specified Automated Cellular Image Analysis System: A Clinicopathologic Study. Saudi J Gastroenterol. 2012; 18(4):268–276.
35.Claudio TR, Tanaka S, Haruma K, Yoshihara M, Sumii K, Kajiyama G. Proliferating Cell Nuclear Antigen Expression at The Invasive Tumor Margin Predicts Malignant Potential of Colorectal Carcinomas. Cancer. 1994; 73(3):575–579.
36.Liu R, Sun K, Wang Y, Jiang Y, Kang J, Ma H. The Effects of Proliferating Cell Nuclear Antigen and P53 in Patients with Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Annals Transl Med. 2021; 9(23):1739–1739.
37.Zhou H, Huang T, Xiong Y, Peng L, Wang R, Jun Zhang G. (2018). The Prognostic Value of Proliferating Cell Nuclear Antigen Expression in Colorectal Cancer: A Meta-analysis. Medicine (Baltimore). 2018; 97(50): e13625.
38.Nakae S, Nakamura T, Ikegawa R, Yoshioka H, Shirono J, Tabuchi Y. Evaluation of Argyrophilic Nucleolar Organizer Region and Proliferating Cell Nuclear Antigen in Colorectal Cancer. J Surg Oncol. 1998; 69(1):28–35.
39.Hajrezaie M, Hassandarvish P, Moghadamtousi SZ, Gwaram NS, Golbabapour S, Hussien AN, Almagrami AA, Zahedifard M, Rouhollahi E, Karimian H, Fani S, Kamalidehghan B, Majid NA, Ali HM, Abdulla MA. Chemopreventive Evaluation of a Schiff Base Derived Copper (II) Complex Against Azoxymethane-Induced Colorectal Cancer in Rats. PLoS One. 2014; 9(3): e91246.
40.Hajrezaie M, Shams K, Moghadamtousi SZ, Karimian H, Hassandarvish P, Emtyazjoo M, Zahedifard M, Majid NA, Ali HM, Abdulla MA. Chemoprevention of Colonic Aberrant Crypt Foci by Novel Schiff Based Dichloride (4-methoxy-2-{[2-(piperazin-4-Ium-1-Yl) ethyl] iminomethyl} phenolate) Cd Complex in Azoxymethane-induced Colorectal Cancer in Rats. Sci Rep. 2015; 23(5):12379.
41.Madka V, Kumar G, Pathuri G, Zhang Y, Lightfoot S, Asch AS, Mohammed A, Steele VE, Rao CV. Bisphosphonates Zometa and Fosamax Synergize with Metformin to Prevent AOM-induced Colon Cancer in F344 Rat Model. Cancer Prev Res. 2020; 13(2):185–194.
42.Moghadamtousi SZ, Rouhollahi E, Karimian H, Fadaeinasab M, Firoozinia M, Ameen Abdulla M, Kadir HA. The Chemopotential Effect of Annona muricata Leaves Against Azoxymethane-induced Colonic Aberrant Crypt Foci in Rats and the Apoptotic Effect of Cetogenin Annomuricin E in HT-29 Cells: A Bioassay-Guided Approach. PloS One. 2015; 10(4): e0122288.
43.Shwter AN, Abdullah NA, Alshawsh MA, El-Seedi HR, Al-Henhena NA, Khalifa SA, Abdulla MA. Chemopreventive Effect of Phaleria Macrocarpa on Colorectal Cancer Aberrant Crypt Foci In Vivo. J Ethnopharmacol. 2016; 4(193):195–206.
44.Xu G, Ren G, Xu X, Yuan H, Wang Z, Kang L, Yu W, Tian K. Combination of Curcumin and Green Tea Catechins Prevents Dimethylhydrazine-Induced Colon Carcinogenesis. Food Chem Toxicol. 2010; 48(1):390-395.
45.Ahmed KA, Shareef SH, Faraj TA, Abdulla MA, Najmaldin SK, Agha NFS, Kheder RK. Chemoprotective Effect of Arbutin on Azoxymethane-induced Aberrant Crypt Foci in Rat Colon Via Modulation of PCNA/Bax Protein. Braz Journal Med Biol Res. 2024; 1(57): 13306-13316.
46.Kilari BP, Kotakadi VS, Penchalaneni J. Anti-Proliferative and Apoptotic Effects of Basella Rubra (L.) Against 1,2-Dimethylhydrazine-Induced Colon Carcinogenesis in Rats. Asian Pac J Cancer Prev. 2016; 17(1):73–80.
47.Zakaria ZA, Nor Hazalin NAM, Zaid SNHM, Ghani MA, Hassan MH, Gopalan HK, Sulaiman MR. Antinociceptive, anti-inflammatory and antipyretic effects of Muntingia calabura aqueous extract in animal models. J of Nat Med. 2007; 61(4): 443–448.
48.Nguyen NVT, Duong N, Nguyen KH, Bui N, Pham T, Nguyen KT, Le PH, Kim K. Effect of Extraction Solvent on Total Phenol, Flavonoid Content, and Antioxidant Activity of Avicennia Officinalis. Biointerface Res Appl Chem. 2021; 12, 2678-2690.
49.Jisha N, Vysakh A, Vijeesh V, Anand PS, Latha MS. Methanolic Extract of Muntingia Calabura Mitigates 1,2-Dimethyl Hydrazine Induced Colon Carcinogenesis in Wistar Rats. Nutr Cancer. 2021; 73(11–12): 2363–2375.
50.Almagrami AA, Alshawsh MA, Saif-Ali R, Shwter A, Salem SD, Abdulla MA. Evaluation of Chemopreventive Effects of Acanthus ilicifolius against Azoxymethane-Induced Aberrant Crypt Foci in the Rat Colon. PLoS One. 2014; 9(5): e96004.
51.Nasir NLM, Kamsani NE, Mohtarrudin N, Othman F, Tohid SFM, Zakaria ZA, Md Nasir NL, Kamsani NE, Mohtarrudin N, Othman F, Md. Tohid SF, Zakaria ZA. Anticarcinogenic activity of Muntingia calabura leaves methanol extract against the azoxymethane-induced colon cancer in rats involved modulation of the colonic antioxidant system partly by flavonoids. Pharm Biol. 2017; 55(1): 2102–2109.
52.Subarmaniam T, Mahmad Rusli RN, Perumal KV, Yong YK, Hadizah S, Othman F, Salem K, Shafie NH, Hasham R, Yin KB, Abdul Kadir KK, Bahari H, Zakaria ZA. The Potential Chemopreventive Effect of Andrographis paniculata on 1,2-Dimethylhydrazine and High-Fat-Diet-Induced Colorectal Cancer in Sprague Dawley Rats. Int J Mol Sci. 2023; 24(6)5224-5241.
53.Bird RP. Role of aberrant crypt foci in understanding the pathogenesis of colon cancer. Cancer Lett. 1995; 93(1): 55–71.
54.Norazalina S, Norhaizan ME, Hairuszah I, Norashareena MS. Anticarcinogenic efficacy of phytic acid extracted from rice bran on azoxymethane-induced colon carcinogenesis in rats. Exp Toxicol Pathol. 2010; 62(3): 259–268.
55.Fenoglio-Preiser CM, Noffsinger AE, Stemmermann GN, Lantz PE, Isaacson PG. Gastrointestinal pathology: An Atlas and Text. 3rd ed. Lippincott Williams and Wilkins. 2008.
56.Compton CC, Greene FL. The staging of colorectal cancer: 2004 and beyond. CA Cancer J Clin. 2004; 54(6): 295-308.
57.Umesalma S, Nagendraprabhu P, Sudhandiran G. Antiproliferative and apoptotic-inducing potential of ellagic acid against 1,2-dimethyl hydrazine-induced colon tumorigenesis in Wistar rats. Mol Cell Biochem. 2014; 388(1–2): 157–172
58.Wani AK, Akhtar N, Mir TG, Singh R, Jha PK, Mallik SK, Sinha S, Tripathi SK, Jain A, Jha A, Devkota HP, Prakash A. Targeting Apoptotic Pathway of Cancer Cells with Phytochemicals and Plant-Based Nanomaterials. Biomolecules. 2023; 13(2): 194-228.
59.Pucci B, Kasten M, Giordano A. Cell Cycle and Apoptosis. Neoplasia. 2000; 2(4): 291-299.
60.Tanaka, T. Colorectal carcinogenesis and oxidative stress: Role of natural antioxidants. Cancer Lett. 2020; 475: 88–96.
61.Saufi SM, Mastuki SN, Faudzi SMM, Saad N. Bioactive constituents of Muntingia calabura and their biological properties. Plants. 2021;10(4): 812.
62.Tengku MTS, Zainal ANI, Zainol MI, Ismail N. Phytochemicals as regulators of signaling pathways in colon cancer: A mechanistic review. Front Pharmacol. 2020;13, 902175.
63.Atanasov AG, Zotchev SB, Dirsch VM, Supuran CT. Natural products in drug discovery: advances and opportunities. Nat Rev Drug Discov. 2021; 20(3): 200–216.
64.Elmore S. Apoptosis: A review of programmed cell death. Toxicol Pathol. 2007; 35(4): 495–516.
65.Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9(1):47–59.
66.Zakaria ZA, Mohd SMH, Cheema MS, Kader AA, Teh LK, Salleh MZ. Antinociceptive activity of methanolic extract of Muntingia calabura leaves: further elucidation of the possible mechanisms. BMC Complement Altern Med. 2014;14:63.
67.Zakaria ZA, Rofiee MS, Mohamed AM, Teh LK, Salleh MZ, Sulaiman MR. In vitro antiproliferative and antioxidant activities and total phenolic contents of the extracts of Melastoma malabathricum leaves. J Acupunct Meridian Stud. 2011; 4(4): 248–256.
68.Yang C, Xie X, Tang H, Dong X, Zhang X, Huang F. Transcriptome analysis reveals GA induced apoptosis in HCT116 human colon cancer cells through calcium and p53 signal pathways. RSC Adv. 2018; 8:12449-12458
69.Wang A, Yoshimi N, Ino N, Tanaka T, Mori H. “Overexpression of cyclin B1 in human colorectal cancers.” J Cancer Res Clin Oncol. 1997;123(2):124–127.
70.Perillo B, Di Donato M, Pezone A, Erika Di Z, Pia G, Giovanni G, Gabriella C, Antimo M. ROS in cancer therapy: The bright side of the moon. Exp Mol Med. 2020; 52(2): 192–203.
71.Takahashi M, Wakabayashi K. Gene mutations and altered gene expression in azoxymethane-induced colon carcinogenesis in rodents. Cancer Sci. 2004; 95(6): 475–480.
72.Rahman MM, Fakir MSA, Rahman MM. Antioxidant and anticancer activities of Muntingia calabura. Pharm Biol. 2020; 58(1): 1–12.
73.Perše M. Oxidative stress in the pathogenesis of colorectal cancer: cause or consequence? Biomed Res Int. 2013; 725710