LC-HRMS Profiling, Total Flavonoid and Phenolic Content of Brown Algae Originating from the Southern Coast of Indonesia
Main Article Content
Abstract
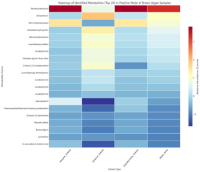
Brown algae (Sargassum polycystum) are widely distributed in Indonesian marine ecosystems. Despite their abundance, pharmaceutical exploration remains limited compared to red and green algae. This study aimed to profile metabolites and evaluate the total phenolic content (TPC) and total flavonoid content (TFC) of brown algae collected from Indonesia’s southern coast using liquid chromatography-high-resolution mass spectrometry (LC-HRMS). Powdered samples of brown algae were extracted separately by maceration using three different polarity-based solvents: ethanol, ethyl acetate, and n-hexane. The extracts were analysed for TPC and TFC using Folin ciocalteu and aluminium chloride colorimetric assays, respectively. The metabolites profile of the extracts was analysed using LC-HRMS. Ethyl acetate extract exhibited the highest TPC (48.570 ± 0.008 mg GAE/g), while n-Hexane extract showed the highest TFC (85.271 ± 0.017 mg QE/g). LC-HRMS profiling identified 232-328 metabolites across the extracts, with n-hexane yielding the greatest chemical diversity. Key bioactive compounds identified included Xestoaminol C (highest in ethanol extract at 5.57%), Ceramides (13.46% of total content in ethanol extract), and Fucoxanthin (exclusive to ethanol extract, 0.52%). These metabolites are known for their anticancer, neuroprotective, antioxidant and antimicrobial properties. Heatmap analysis of the top 20 metabolites revealed solvent-dependent clustering patterns, highlighting the influence of extraction methods on metabolites recovery. The findings underscore the pharmaceutical and nutraceutical potential of Indonesian brown algae and demonstrate the invaluable role of LC-HRMS for marine natural product research.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.El-Gammal MI, Abou-Dobara MI, Ibrahim HAH, Abdulhafith SA, Okbah MA. Polyphenols in Selected Marine Algae and Aromatic Herbs with Antimicrobial Properties: A Comparative Study. Egypt J Aqua Res. 2024; 50(1):71–77. doi:10.1016/j.ejar.2023.12.006.
2.Delgado A, Gonçalves S, Romano A. Mediterranean Diet: The Role of Phenolic Compounds from Aromatic Plant Foods. Foods. 2023; 12(4):840. doi:10.3390/foods12040840.
3.Murdiono WE, Razak NAA, Halmi MIE, Yong JWH, Mahmud K. Optimization of Aqueous Extraction of Phenolic Compounds and Bioactive Profiles from Brown (Sargassum polycystum) and Red (Kappaphycus alvarezii) Seaweeds Using the Response Surface Method. Algal Res. 2025; 86:103924. doi:10.1016/j.algal.2025.103924.
4.Sobuj MKA, Shemul MS, Islam MdS, Islam MdA, Mely SS, Ayon MH. Qualitative and Quantitative Phytochemical Analysis of Brown Seaweed Sargassum polycystum Collected from Bangladesh with Its Antioxidant Activity Determination. Food Chem Adv. 2024; 4:100565. doi:10.1016/j.focha.2023.100565.
5.Dip MdRR, Sobuj MKA, Islam MdS, Akter A, Hasan MdM, Tasnim N. Phytochemicals, Antioxidant and Antibacterial Activity of Crude Extract of Sargassum polycystum Collected from Bangladesh. Food Hum. 2024; 2:100278. doi:10.1016/j.foohum.2024.100278.
6.Alreshidi M, Badraoui R, Adnan M, Patel M, Alotaibi A, Saeed M. Phytochemical Profiling, Antibacterial, and Antibiofilm Activities of Sargassum sp. (Brown Algae) from the Red Sea: ADMET Prediction and Molecular Docking Analysis. Algal Res. 2023; 69:102912. doi:10.1016/j.algal.2022.102912.
7.Meinita MDN, Yulia R, Nursid M, Nurulita NA, Harwanto D, Riviani R. Morpho-Anatomical Characteristics, Phytochemical and Antibacterial Potential of Sargassum polycystum Collected Along the Southern Coast of Java, Indonesia. Biodiversitas. 2024; 25(8):2669-2681. doi:10.13057/biodiv/d250840.
8.Susanti S, Sundari RS, Sarwatiningsih Y, Yuliawati S, Kurniawan R, Mardianingrum R. The Effect of Ultrasound-Assisted Extraction Solvent on Antimicrobial Activity of Gadung Tuber (Dioscorea hispida Dennst.). J Pharmacopolium. 2020; 3(3):144–151. doi:10.36465/jop.v3i3.654.
9.Beniddir MA, Kang K Bin, Genta-Jouve G, Huber F, Rogers S, van der Hooft JJJ. Advances in Decomposing Complex Metabolite Mixtures Using Substructure- and Network-Based Computational Metabolomics Approaches. Nat Prod Rep. 2021; 38(11):1967–1993. doi:10.1039/D1NP00023C.
10.Ningsih R, Rafi M, Tjahjoleksono A, Bintang M, Megia R. Ripe Pulp Metabolite Profiling of Ten Indonesian Dessert Banana Cultivars Using UHPLC-Q-Orbitrap HRMS. Eur Food Res Technol. 2021; 247(11):2821–2830. doi:10.1007/s00217-021-03834-7.
11.Aziz Z, Yuliana ND, Simanjuntak P, Rafi M, Mulatsari E, Abdillah S. Investigation of Yacon Leaves (Smallanthus sonchifolius) for alpha-Glucosidase Inhibitors Using Metabolomics and In Silico Approach. Plant Foods Hum Nutr. 2021; 76(4):487–493. doi:10.1007/s11130-021-00926-3.
12.Umar AH, Ratnadewi D, Rafi M, Sulistyaningsih YC. Untargeted Metabolomics Analysis Using FTIR and UHPLC-Q-Orbitrap HRMS of Two Curculigo Species and Evaluation of Their Antioxidant and alpha-Glucosidase Inhibitory Activities. Metabolites. 2021; 11(1):42. doi:10.3390/metabo11010042.
13.Sinan KI, Saftić L, Peršurić Ž, Pavelić SK, Etienne OK, Picot-Allain MCN. A Comparative Study of the Chemical Composition, Biological and Multivariate Analysis of Crotalaria retusa L. Stem Barks, Fruits, and Flowers Obtained via Different Extraction Protocols. S Afr J Bot. 2020; 128:101–108. doi:10.1016/j.sajb.2019.10.019.
14.Aryal B, Adhikari B, Aryal N, Bhattarai BR, Khadayat K, Parajuli N. LC-HRMS Profiling and Antidiabetic, Antioxidant, and Antibacterial Activities of Acacia catechu (L.f.) Willd. Biomed Res Int. 2021; 2021(1):7588711. doi:10.1155/2021/7588711.
15.Alallam B, Abdulameed HT, Lim V. Unbiased Metabolomic and Chemometric Profiles of Three Sargassum polycystum Extracts Using GCMS and LCMS/MS: Content Analysis,
Correlation Analysis and Molecular Docking. Food Chem. 2025; 470:142666. doi:10.1016/j.foodchem.2024.142666.
16.Irianto I, Agustien N, Ni WT, Astuti A, Kokom K, Lailatul Q, Chaidir C, Ariyanti S, Rika W, Dwila NR, Nicky RP. From Sea to Solution: A Review of Green Extraction Approaches for Unlocking the Potential of Brown Algae. S Afr J Chem Eng. 2024; 48:1–21. doi:10.1016/j.sajce.2024.01.001.
17.Susanti S, Sundari RS, Rizkuloh LR, Mardianingrum R. Effect of Solvents Extraction on Total Phenolic Content and Antioxidant Activity of Gadung Extract (Dioscorea hispida Dennst.). Biopropal Ind. 2021; 12(1):43-49. doi:10.36974/jbi.v12i1.6482.
18.Septiani G, Susanti S, Sucitra F. Effect of Different Extraction Method on Total Flavonoid Contents of Sansevieria trifasciata P. Leaves Extract. J Farm Galenika (Galenika J Pharm) (e-J). 2021; 7(2):143–150. doi:10.22487/j24428744.2021.v7.i2.15573.
19.Supriadi A, Ridhowati S, Saputra D, Wulandari, Lestari SD. Untargeted Metabolomics Profiling for the Geographical Authentication of Traditional Pempek Using High-Resolution Orbitrap Mass Spectrometry. Food Chem Adv. 2025; 6:100914. doi:10.1016/j.focha.2025.100914.
20.Kusuma J, Analianasari, Wahyudi A, Abdullah MK, Hasan AZ, Asrowardi I. Diversity of the Non-Targeted Metabolomic Data Across Various Varieties of Cloves (Syzygium spp.). Data Brief. 2025; 58:111237. doi:10.1016/j.dib.2024.111237.
21.Rivaldi M, Frediansyah A, Aziz SAA, Nugroho AP. Active Biomonitoring of Stream Ecosystems: Untargeted Metabolomic and Proteomic Responses and Free Radical Scavenging Activities in Mussels. Ecotoxicology. 2025; 34(3):425–443. doi:10.1007/s10646-024-02846-9.
22.Dea Firdaus M, Artanti N, Hanafi M, Rosmalena R. Phytochemical Constituents and In vitro Antidiabetic and Antioxidant Properties of Various Extracts of Kenikir (Cosmos caudatus) Leaves. Pharmacogn J. 2021; 13(4):890–895. doi:10.5530/pj.2021.13.114.
23.Rahimmalek M, Afshari M, Sarfaraz D, Miroliaei M. Using HPLC and Multivariate Analyses to Investigate Variations in the Polyphenolic Compounds as well as Antioxidant and Antiglycative Activities of Some Lamiaceae Species Native to Iran. Ind Crops Prod. 2020; 154:112640. doi:10.1016/j.indcrop.2020.112640.
24.Yusoff NAH, Rukayadi Y, Abas F, Khatib A, Hassan M. Antimicrobial Stability of Cosmos caudatus Extract at Varies pH and Temperature, and Compounds Identification for Application as Food Sanitiser. Food Res. 2021; 5(3):83–91. doi:10.26656/fr.2017.5(3).710.
25.Saw NMMT, Suwanchaikasem P, Zuniga-Montanez R, Qiu G, Marzinelli EM, Wuertz S. Influence of Extraction Solvent on Nontargeted Metabolomics Analysis of Enrichment Reactor Cultures Performing Enhanced Biological Phosphorus Removal (EBPR). Metabolites. 2021; 11(5):269. doi:10.3390/metabo11050269.
26.Wiedmaier-Czerny N, Vetter W. LC-Orbitrap-HRMS Method for Analysis of Traces of Triacylglycerols Featuring Furan Fatty Acids. Anal Bioanal Chem. 2023; 415(5):875–885. doi:10.1007/s00216-022-04480-y.
27.Tamoradi T, Kiasat AR, Veisi H, Nobakht V, Karmakar B. RSM Process Optimization of Biodiesel Production from Rapeseed Oil and Waste Corn Oil in the Presence of Green and Novel Catalyst. Sci Rep. 2022; 12(1):19652. doi:10.1038/s41598-022-20538-4.
28.PubChem. Xestoaminol C. National Institutes of Health. 2025. CID 14756407. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/14756407.
29.Steven AN, Bachuri K, Mat Hussin NSS, Mohd Noh NI. Anticancer Agents and Genetic Identification of Pterospermum, an Indigenous Plant of Sarawak, Malaysia. J Taibah Univ Sci. 2024; 18(1):2325677. doi:10.1080/16583655.2024.2325677.
30.Uchida Y, Park K. Ceramides in Skin Health and Disease: An Update. Am J Clin Dermatol. 2021; 22(6):853–866. doi:10.1007/s40257-021-00619-2.
31.Abdelhameed R, Elgawish MS, Mira A, Ibrahim AK, Ahmed SA, Shimizu K. Anti-Choline Esterase Activity of Ceramides from the Red Sea Marine Sponge Mycale euplectellioides. RSC Adv. 2016; 6(24):20422–20430. doi:10.1039/C5RA26424C.
32.Wang X, Zhao M, Xia G, Shi H, Li C, Shen X. A Review of Sphingolipids from Marine Sources and Their Analytical Method, Metabolic Process, and Essential Roles in Human Health. Food Front. 2024; 5(5):2015–2042. doi:10.1002/fft2.450.
33.Sugawara T. Sphingolipids as Functional Food Components: Benefits in Skin Improvement and Disease Prevention. J Agric Food Chem. 2022; 70(31):9597–9609. doi:10.1021/acs.jafc.2c01731.
34.Mohibbullah Md, Haque MdN, Sohag AAM, Hossain MdT, Zahan MdS, Uddin MdJ. A Systematic Review on Marine Algae-Derived Fucoxanthin: An Update of Pharmacological Insights. Mar Drugs. 2022; 20(5):279. doi:10.3390/md20050279.
35.Li Y, Kim MB, Park YK, Lee JY. Fucoxanthin Metabolites Exert Anti-Fibrogenic and Antioxidant Effects in Hepatic Stellate Cells. J Agric Food Res. 2021; 6:100245. doi:10.1016/j.jafr.2021.100245.
36.Yang YP, Tong QY, Zheng SH, Zhou MD, Zeng YM, Zhou TT. Anti-Inflammatory Effect of Fucoxanthin on Dextran Sulfate Sodium-Induced Colitis in Mice. Nat Prod Res. 2020; 34(12):1791–1795. doi:10.1080/14786419.2018.1528593.
37.Wang J, Ma Y, Yang J, Jin L, Gao Z, Xue L. Fucoxanthin Inhibits Tumour-Related Lymphangiogenesis and Growth of Breast Cancer. J Cell Mol Med. 2019; 23(3):2219–2229. doi:10.1111/jcmm.14151.
38.Hitoe S and Shimoda H. Seaweed Fucoxanthin Supplementation Improves Obesity Parameters in Mild Obese Japanese Subjects. Funct Foods Health Dis. 2017; 7(4):246-262. doi:10.31989/ffhd.v7i4.333.
39.Rodríguez-Luna A, Ávila-Román J, González-Rodríguez ML, Cózar MJ, Rabasco AM, Motilva V. Fucoxanthin-Containing Cream Prevents Epidermal Hyperplasia and UVB-Induced Skin Erythema in Mice. Mar Drugs. 2018; 16(10):378. doi:10.3390/md16100378.
40.Karpiński TM and Adamczak A. Fucoxanthin—An Antibacterial Carotenoid. Antioxidants. 2019; 8(8):239. doi:10.3390/antiox8080239.