Cytotoxic Effects of Crassocephalum crepidioides Leaf Extract on T47D Cells: A Network Pharmacology Approach
Main Article Content
Abstract
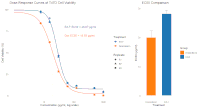
Crassocephalum crepidioides, locally known as Sintrong, is a medicinal plant extensively utilized in traditional Southeast Asian medicine. Despite the potential, its pharmacological properties, particularly anticancer effects, remain insufficiently studied. Therefore, this study aims to evaluate the cytotoxic effects of Crassocephalum crepidioides ethyl acetate leaf fraction on T47D luminal breast cancer cells and to elucidate the underlying mechanisms through an integrated experimental and network pharmacology approach. Phytochemical screening and MTT cytotoxicity assays were conducted on the ethyl acetate fraction. Computational analyses included target prediction using SwissTargetPrediction, protein-protein interaction (PPI) mapping with STRING, and functional enrichment analysis through WebGestalt. The results showed that the extract had significant cytotoxicity, with EC₅₀ value of 28.07 ± 0.67 μg/mL, comparable to doxorubicin. Phytochemical profiling revealed a high abundance of alkaloids, terpenoids, and phenolics. In addition, network pharmacology analysis identified 3 major compounds predicted to target PTGS1 (COX-1), PPARA (PPAR-α), and CNR2 (CB2). Enrichment analysis implicated pathways related to cancer, including PPAR signalling and the modulation of inflammatory responses. These results suggest that anticancer effects of Sintrong involve a novel multi-target mechanism associated with lipid metabolism and the endocannabinoid system, exhibiting selective toxicity toward cancer cells. This current study provides comprehensive scientific validation of Sintrong's potential as a source of anticancer agents for luminal breast cancer. However, further studies are necessary to isolate active compounds, evaluate in vivo efficacy and safety, and explore potential synergistic effects with conventional therapies.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. F Bray 1, M Laversanne, H Sung, J Ferlay, RL Siegel, I Soerjomataram, A Jemal. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229–63. Doi:10.3322/caac.21834
2. Yunmeng Z, Yuting J, Siwen L, Jingjing L, Jie W, Qianyun J, Xiaomin L, Hongyuan D, Zhuowei F, Ya L, Yacong Z, Zhangyan L, Fangfang S, Fengju S, Lei Y, Hong L, and Yubei Huang . Global burden of female breast cancer: new estimates in 2022, temporal trend and future projections up to 2050 based on the latest release from GLOBOCAN. Journal of the National Cancer Center. 2025; 5 (3): 287-296. Doi: 10.1016/j.jncc.2025.02.002.
3. Mohammadpour S, Soleimanpour S, Javan-Noughabi J, Gallehzan NA, Aboutorabi A, Jahangiri R. A systemmatic literature review on indirect costs of women with breast cancer. Cost Effectiveness and Resource Allocation. 2022 ;20(1):68. Doi:10.1186/s12962-022-00408-6Doi:10.1186/s12962-022-00408-6
4. Duggan C, Trapani D, Ilbawi AM, Fidarova E, Laversanne M, Curigliano G, F Bay, BO Anderson. National health system characteristics, breast cancer stage at diagnosis, and breast cancer mortality: a population-based analysis. Lancet Oncol. 2021;22(11):1632–42. Doi:10.1016/S1470-2045(21)00462-9Doi:10.1016/S1470-2045(21)00462-9
5. Obidiro O, Battogtokh G, Akala EO. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics. 2023;15(7):1796. Doi:10.3390/pharmaceutics15071796Doi:10.3390/pharmaceutics15071796
6. Mills JN, Rutkovsky AC, Giordano A. Mechanisms of resistance in estrogen receptor positive breast cancer: overcoming resistance to tamoxifen/aromatase inhibitors. Curr Opin Pharmacol. 2018;41:59–65. Doi:10.1016/j.coph.2018.04.009Doi:10.1016/j.coph.2018.04.009
7. Shaikh AY, Shih JA. Chemotherapy-Induced Cardiotoxicity. Curr Heart Fail Rep. 2012; 9(2):117–27. https://pubmed.ncbi.nlm.nih.gov/24023601/https://pubmed.ncbi.nlm.nih.gov/24023601/
8. Yang J, Chen WY, Fu Y, Yang T, Luo XD, Wang YH. Medicinal and edible plants used by the Lhoba people in Medog County, Tibet, China. J Ethnopharmacol. 2020; 249:112430. Doi:10.1016/j.jep.2019.112430Doi:10.1016/j.jep.2019.112430
9. Zhang S, Liu K, Liu Y, Hu X, Gu X. The role and application of bioinformatics techniques and tools in drug discovery. Front Pharmacol. 2025;16. Doi:10.3389/fphar.2025.1547131Doi:10.3389/fphar.2025.1547131
10. Yulyana A, Amin C, Simanjuntak P, Abdillah S, Rohman A, Mugiyanto E. Assessing the Antimetabolite Activity of Anthocyanins in Cantigi Fruits from Two Conservation Sites in Indonesia. Indonesian Journal of Pharmacy. 2023; 34(3), 450–459. Doi:10.22146/ijp.8788
11. Falodun, A., Uzoekwe A. S., and Shengxiang Q. Phytochemical, Anticancer and Antioxidant Evaluation of Potential Chemical Consituents of Calliandria Surinamensis. Nig J. Biotech. Vol. 21 (2010) 55 – 59 . Doi: 10.4314/njb.v21i1
12. Mohanraj K, Karthikeyan BS, Vivek-Ananth RP, Chand RPB, Aparna SR, Mangalapandi P. IMPPAT: A curated database of Indian Medicinal Plants, Phytochemistry And Therapeutics. Sci Rep. 2018;8(1):4329. Doi:10.1038/s41598-018-22631-zDoi:10.1038/s41598-018-22631-z
13. McWilliam H, Li W, Uludag M, Squizzato S, Park YM, Buso N,. Analysis Tool Web Services from the EMBL-EBI. Nucleic Acids Res. 2013;41(W1):W597–600. Doi:10.1093/nar/gkt376Doi:10.1093/nar/gkt376
14. Zoete V, Daina A, Bovigny C, Michielin O. SwissSimilarity: A Web Tool for Low to Ultra High Throughput Ligand-Based Virtual Screening. J Chem Inf Model. 2016;56(8):1399–404. Doi:10.1021/acs.jcim.6b00174
15. Szklarczyk D, Nastou K, Koutrouli M, Kirsch R, Mehryary F, Hachilif R,. The STRING database in 2025: protein networks with directionality of regulation. Nucleic Acids Res. 2025;53(D1):D730–7. Doi:10.1093/nar/gkae1113Doi:10.1093/nar/gkae1113
16. Mugiyanto E, Adikusuma W, Irham LM, Huang WC, Chang WC, Kuo CN. Integrated genomic analysis to identify druggable targets for pancreatic cancer. Front Oncol. 2022;12. Doi:0.3389/fonc.2022.989077Doi:0.3389/fonc.2022.989077
17. Kanehisa M, Furumichi M, Sato Y, Matsuura Y, Ishiguro-Watanabe M. KEGG: biological systems database as a model of the real world. Nucleic Acids Res. 2025;53(D1):D672–7. Doi:10.1093/nar/gkae909Doi:10.1093/nar/gkae909
18. Liao Y, Wang J, Jaehnig EJ, Shi Z, Zhang B. WebGestalt 2019: gene set analysis toolkit with revamped UIs and APIs. Nucleic Acids Res. 2019 ;47(W1):W199–205. Doi:10.1093/nar/gkz401Doi:10.1093/nar/gkz401
19. Elizarraras JM, Liao Y, Shi Z, Zhu Q, Pico AR, Zhang B. WebGestalt 2024: faster gene set analysis and new support for metabolomics and multi-omics. Nucleic Acids Res. 2024;52(W1):W415–21. Doi:10.1093/nar/gkae456Doi:10.1093/nar/gkae456
20. Kongsaeree P, Prabpai S, Sriubolmas N, Vongvein C, Wiyakrutta S. Antimalarial Dihydroisocoumarins Produced by Geotrichum sp., an Endophytic Fungus of Crassocephalum c repidioides. J Nat Prod. 2003;66(5):709–11. Doi:10.1021/np0205598Doi:10.1021/np0205598
21. Hegazy MEF, Ohta S, Abdel-latif FF, Albadry HA, Ohta E, Paré PW,. Cyclooxygenase (COX)-1 and -2 Inhibitory Labdane Diterpenes from Crassocephalum mannii. J Nat Prod. 2008;71(6):1070–3. Doi:10.1021/np800017xDoi:10.1021/np800017x
22. Zollo PHA, Kuiate JR, Menut C, Bessiere JM. Aromatic Plants of Tropical Central Africa. XXXVI. Chemical Composition of Essential Oils from Seven Cameroonian Crassocephalum Species. Journal of Essential Oil Research. 2000;12(5):533–6. Doi:10.1080/10412905.2000.9712152Doi:10.1080/10412905.2000.9712152
23. Fidyt K, Fiedorowicz A, Strządała L, Szumny A. β ‐caryophyllene and β ‐caryophyllene oxide—natural compounds of anticancer and analgesic properties. Cancer Med. 2016;5(10):3007–17. Doi:10.1002/cam4.816Doi:10.1002/cam4.816
24. Blücher C, Stadler SC. Obesity and Breast Cancer: Current Insights on the Role of Fatty Acids and Lipid Metabolism in Promoting Breast Cancer Growth and Progression. Front Endocrinol (Lausanne). 2017;8. Doi:10.3389/fendo.2017.00293Doi:10.3389/fendo.2017.00293
25. Yao P, Liu Y. Terpenoids: Natural Compounds for Non-Alcoholic Fatty Liver Disease (NAFLD) Therapy. Molecules. 2022;28(1):272. Doi:10.3390/molecules28010272Doi:10.3390/molecules28010272
26. Pérez-Gómez E, Andradas C, Blasco-Benito S, Caffarel MM, García-Taboada E, Villa-Morales M,. Role of Cannabinoid Receptor CB2 in HER2 Pro-oncogenic Signaling in Breast Cancer. J Natl Cancer Inst. 2015;107(6). Doi:10.1093/jnci/djv077Doi:10.1093/jnci/djv077
27. Ellert-Miklaszewska A, Grajkowska W, Gabrusiewicz K, Kaminska B, Konarska L. Distinctive pattern of cannabinoid receptor type II (CB2) expression in adult and pediatric brain tumors. Brain Res. 2007;1137:161–9. Doi:10.1016/j.brainres.2006.12.060Doi:10.1016/j.brainres.2006.12.060
28. Toyang NJ, Verpoorte R. A review of the medicinal potentials of plants of the genus Vernonia (Asteraceae). J Ethnopharmacol. 2013;146(3):681–723. Doi:10.1016/j.jep.2013.01.040Doi:10.1016/j.jep.2013.01.040
29. Kalač P, Kaltner F. Pyrrolizidine alkaloids of European Senecio/Jacobaea species in forage and their carry-over to milk: A review. Anim Feed Sci Technol. 2021;280:115062. Doi:10.1016/j.anifeedsci.2021.115062Doi:10.1016/j.anifeedsci.2021.115062
30. Casado N, Morante-Zarcero S, Sierra I. The concerning food safety issue of pyrrolizidine alkaloids: An overview. Trends Food Sci Technol. 2022;120:123–39 . Doi:10.1016/j.tifs.2022.01.007Doi:10.1016/j.tifs.2022.01.007
31. Fu PP, Xia Q, Lin G, Chou MW. Pyrrolizidine Alkaloids—Genotoxicity, Metabolism Enzymes, Metabolic Activation, and Mechanisms. Drug Metab Rev. 2004;36(1):1–55. Doi:10.1081/DMR-120028426Doi:10.1081/DMR-120028426
32. Li N, Xia Q, Ruan J, P. Fu P, Lin G. Hepatotoxicity and Tumorigenicity Induced by Metabolic Activation of Pyrrolizidine Alkaloids in Herbs. Curr Drug Metab. 2011;12(9):823–34. Doi:10.2174/138920011797470119Doi:10.2174/138920011797470119
33. Chojkier M. Hepatic sinusoidal-obstruction syndrome: toxicity of pyrrolizidine alkaloids. J Hepatol. 2003;39(3):437–46. Doi:10.1016/S0168-8278(03)00231-9Doi:10.1016/S0168-8278(03)00231-9
34. Zhuge Y, Liu Y, Xie W, Zou X, Xu J, Wang J. Expert consensus on the clinical management of pyrrolizidine alkaloid‐induced hepatic sinusoidal obstruction syndrome. J Gastroenterol Hepatol. 2019;34(4):634–42. Doi:10.1111/jgh.14612Doi:10.1111/jgh.14612
35. Aryal B, Raut BK, Bhattarai S, Bhandari S, Tandan P, Gyawali K,. Potential Therapeutic Applications of Plant-Derived Alkaloids against Inflammatory and Neurodegenerative Diseases. Evid Based Complement Alternat Med. 2022;2022:1–18. Doi:10.1155/2022/7299778Doi:10.1155/2022/7299778
36. Molyneux RJ, Gardner DL, Colegate SM, Edgar JA. Pyrrolizidine alkaloid toxicity in livestock: a paradigm for human poisoning? Food Additives & Contaminants: Part A. 2011;28(3):293–307. Doi:10.1080/19440049.2010.547519Doi:10.1080/19440049.2010.547519
37. Popov SA, Sheremet OP, Kornaukhova LM, Grazhdannikov AE, Shults EE. An approach to effective green extraction of triterpenoids from outer birch bark using ethyl acetate with extractant recycle. Ind Crops Prod. 2017;102:122–32. Doi:10.1016/j.indcrop.2017.03.020Doi:10.1016/j.indcrop.2017.03.020
38. Boobis A, Gundert-Remy U, Kremers P, Macheras P, Pelkonen O. In silico prediction of ADME and pharmacokinetics. Eur J Pharm Sci. 2002;17(4–5):183–93. Doi:10.1016/S0928-0987(02)00185-9Doi:10.1016/S0928-0987(02)00185-9