Evaluation of the Bioactive Potential of Cassia fistula Stem Bark Exhibiting Antioxidant, Antimicrobial, Anti-inflammatory, and Cytotoxic Activities
Main Article Content
Abstract
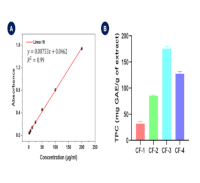
Cassia fistula is a medicinal plant known for its wide range of traditional applications. The present study evaluated the bioactive potential of C. fistula stem bark (CFSB) extracts through antioxidant, antimicrobial, anti-inflammatory, and cytotoxic assays. Sequential extraction with n-hexane (CF-1), chloroform (CF-2), ethyl acetate (CF-3), and methanol (CF-4) was performed to yield distinct crude fractions. Antioxidant properties, total phenolic content (TPC), total flavonoid content (TFC), total antioxidant capacity (TAC), cytotoxic potential, antimicrobial, and in vitro anti-inflammatory activities of the extracts were determined. The CF-3 displayed significant antioxidant activity (IC50 = 8.45 µg/mL), close to standard ascorbic acid (IC50 = 6.54 µg/mL), aligning with TAC results. Also, it demonstrated higher TPC and TFC values, emphasizing its robust antioxidant potential. Cytotoxicity analysis revealed moderate activity in the CF-1 (LC50 = 30.70 µg/mL) compared to vincristine sulfate (LC50 = 3.50 µg/mL). The antimicrobial assay showed that CF-2 and CF-3 exhibited mild to moderate inhibition against Gram-positive bacteria (Bacillus megaterium and Staphylococcus aureus) and the CF-3 was also active against Gram-negative bacteria (Salmonella typhi, and Escherichia coli), but CF-2 was inactive against these strains. Mild antifungal activity against Aspergillus niger was observed for CF-2 and CF-3, while all extracts were inactive against Aspergillus flavus. In vitro protein denaturation studies demonstrated moderate anti-inflammatory activity by CF-4, comparable to diclofenac sodium. These findings suggest that different extracts of CFSB hold promise sources of bioactive compounds with antioxidant, antimicrobial, and anti-inflammatory potential. Further studies may facilitate the isolation of these compounds for potential therapeutic applications.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.Süntar I. Importance of ethnopharmacological studies in drug discovery: role of medicinal plants. Phytochem Rev. 2020; 19(5): 1199–1209.
2.Les F, Cásedas G, López V. Bioactivity of Medicinal Plants and Extracts. J Biology. 2021; 10(7): 220-229.
3.Aslam MS, Ahmad MS. Worldwide Importance of Medicinal Plants: Current and Historical Perspectives. Recent Adv Biol Med. 2016; 2: 88-100.
4.Traditional medicine has a long history of contributing to conventional medicine and continues to hold promise.” Accessed: Nov. 12, 2024. [Online]. Available: https://www.who.int/news-room/feature-stories/detail/traditional-medicine-has-a-long-history-of-contributing-to-conventional-medicine-and-continues-to-hold-promise
5.W. H. Organization, I. U. for C. of N. and N. Resources, and W. W. F. for Nature, Guidelines on the conservation of medicinal plants. Gland: International Union for Conservation of Nature and Natural Resources, 1993. Accessed: Nov. 12, 2024. [Online]. Available: https://iris.who.int/handle/10665/41651
6.Soetan KO, Aiyelaagbe OO. The need for bioactivity-safety evaluation and conservation of medicinal plants - A review. J Med Plants Res. 2009; 3(5): 324-328.
7.Ed-Dahmani I. Phytochemical, Antioxidant activity, and toxicity of wild medicinal plant of Melitotus albus extracts, in vitro and in silico approaches. ACS Omega. 2024; 9(8): 9236–9246.
8.Ling LT, Radhakrishnan AK, Subramaniam T, Cheng HM, Palanisamy PD. Assessment of antioxidant capacity and cytotoxicity of selected Malaysian plants. Molecules. 2010; 15(4): 120-128.
9.Sammar M, Abu‑Farich B, Rayan I, Falah M, Rayan M. Correlation between cytotoxicity in cancer cells and free radical‑scavenging activity: In vitro evaluation of 57 medicinal and edible plant extracts. Oncol Lett. 2019; 18(6): 6563–6571.
10.Vaou N, Stavropoulou E, Voidarou C, Tsigalou C, Bezirtzoglou E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms. 2021; 9(10): 228-236.
11.Maione F, Russo R, Khan H, Mascolo N. Medicinal plants with anti-inflammatory activities. Nat Prod Res. 2016; 30(12): 1343–1352.
12.Shrikant N, Malpani, Manjunath KP. Antidiabetic activity and phytochemical investigations of Cassia fistula Linn. bark. Int J Pharm Sci Res. 2012; 46: 1822-1825.
13.Mazumder UK, Gupta M, Rath N. CNS activities of Cassia fistula in mice. Phytother Res. 1998; 12(7): 520–522.
14.Phongpaichit S, Pujenjob N, Rukachaisirikul V, Ongsakul M. Antifungal activity from leaf extracts of Cassia alata L., Cassia fistula L. and Cassia tora L. Songklanakarin J Sci Technol. 2004; 26(5):741-748.
15.Duraipandiyan V, Ignacimuthu S. Antibacterial and antifungal activity of Cassia fistula L.: an ethnomedicinal plant. J Ethnopharmacol. 2007; 112(3): 590–594.
16.Abo K, Lasaki S, Adeyemi A. Laxative and antimicrobial properties of Cassia species growing in Ibadan. Niger J Nat Prod Med. 1999; 3(1): 47–50.
17.Navanath MS, Naikwade N, Mule S, Krishna P. Evaluation of anti-inflammatory activity of Cassia fistula and Ficus benghalensis. J Pharm Res. 2009; 23(2): 123-129.
18.Kalantari H. Protective effect of Cassia fistula fruit extract on bromobenzene-induced nephrotoxicity in mice. Hum Exp Toxicol. 2011; 30(10): 1710–1715.
19.Siddhuraju P, Mohan PS, Becker K. Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.): a preliminary assessment of crude extracts from stem bark, leaves, flowers and fruit pulp. Food Chem. 2002; 79(1): 61–67.
20.Manonmani G, Bhavapriya V, Kalpana S, Govindasamy S, Apparanantham T. Antioxidant activity of Cassia fistula (Linn.) flowers in alloxan induced diabetic rats. J Ethnopharmacol. 2005; 97(1): 39–42.
21.Gupta M, Mazumder UK, Rath N, Mukhopadhyay DK. Antitumor activity of methanolic extract of Cassia fistula L. seed against Ehrlich ascites carcinoma. J Ethnopharmacol. 2000; 72(1–2): 151–156.
22.Bhakta T. Evaluation of hepatoprotective activity of Cassia fistula leaf extract. J Ethnopharmacol. 1999; 66(3): 277–282.
23.Silawat N, Jarald E, Jain N, Yadav A, Deshmukh P, Nahata B. The mechanism of hypoglycemic and antidiabetic action of hydroalcholic extract of Cassia fistula Linn. in rats. Pharma research. 2009; 1: 113-124.
24.Mwangi RW, Macharia JN, Wagara IN, Bence RL. The medicinal properties of Cassia fistula L: A review. Biomed Pharmacother. 2021; 144: 112-240.
25.Gulcin I and Alwasel SH. DPPH radical scavenging assay. Processes. 2023; 11(8): 12-23.
26.Uddin MF, Das AK, Saha K. Antioxidant and cytotoxic activities of stem and root extracts of Catharanthus roseus cultivated in Bangladesh. Trop J Nat Prod Res. 2022; 6(9): 245-251.
27.Locatelli M, Gindro R, Travaglia F, Coïsson JD, Rinaldi M, Arlorio M. Study of the DPPH-scavenging activity: Development of a free software for the correct interpretation of data. Food Chem. 2009; 114(3): 889–897.
28.Martinez-Morales F, Alonso-Castro AJ, Zapata-Morales JR, Carranza-Álvarez C, Aragon-Martinez OH. Use of standardized units for a correct interpretation of IC50 values obtained from the inhibition of the DPPH radical by natural antioxidants. Chem Pap. 2020; 74(10): 3325–3334.
29.Saeed N, Khan MR, Shabbir M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement Altern Med. 2012; 12(1): 221-231.
30.Rahman M, Das A, Uddin MF, Saha K. Antioxidant and cytotoxicity study on the successive extracts of leaves, stems, and roots of the medicinal plant Garcinia cowa growing in Bangladesh. World J Pharm Res. 2023; 27: 134-145.
31.Kupina S, Fields C, Roman MC, Brunelle BL. Determination of total phenolic content using the Folin-C assay: Single-laboratory validation, First Action 2017.13. J AOAC Int. 2018; 101(5): 1466–1472.
32.Chang CC, Yang MH, Wen HM, Chern JC. Estimation of total flavonoid content in propolis by two complementary colourimetric methods. J Food Drug Anal. 2002; 10(3): 110-124.
33.Prieto P, Pineda M, Aguilar M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: Specific application to the determination of vitamin E. Anal Biochem. 1999; 269(2): 337–341.
34.Rice SA, Maness IB. Brine Shrimp Bioassays: A useful technique in biological investigations. Am Biol Teach. 2004; 66(3): 208–215.
35.Yang X. Antimicrobial susceptibility testing of Enterobacteriaceae: determination of disk content and Kirby-Bauer breakpoint for ceftazidime/avibactam. BMC Microbiol. 2019; 19(1): 240-256.
36.Çadirci E, Sulemyan H, Gurbuz P, Guvenalp Z, Demirezer Z. Anti-inflammatory effects of different extracts from three Salvia species. Turk J Biol. 2012; 36(1): 59–64.
37.Chatterjee P, Chandra S, Dey P, Bhattacharya S. Evaluation of anti-inflammatory effects of green tea and black tea: A comparative: in vitro: study. J Adv Pharm Technol Res. 2012; 3(2): 136-145.
38.Javanmardi J, Stushnoff C, Locke E, Vivanco JM. Antioxidant activity and total phenolic content of Iranian Ocimum accessions. Food Chem. 2003; 83(4): 547–550.
39.Tristantini D, Amalia R. Quercetin concentration and total flavonoid content of anti-atherosclerotic herbs using aluminum chloride colourimetric assay. AIP Conf Proc. 2019; 1: 210-223.
40.Zhao G, Hui Y, Rupprecht JK, McLaughlin JK, Wood KV. Additional bioactive compounds and trilobacin, a novel highly cytotoxic acetogenin, from the bark of Asimina triloba. J Nat Prod. 1992; 55(3): 347–356.
Chaiya P, Senarat S, Phaechamud T, Narakornwit W. In vitro anti-inflammatory activity using thermally inhibiting protein denaturation of egg albumin and antimicrobial activities of some organic solvents. Mater Today Proc. 2022; 65: 2290–2295.