Immunomodulatory Effects of Launaea sarmentosa Extract on Cyclophosphamide-Induced Immunosuppressed Mice
Main Article Content
Abstract
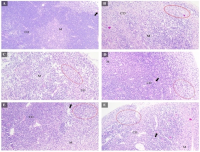
Traditional medicine and herbal extracts offer potential for enhancing immune responses. This study investigated the immunomodulatory effects of Launaea sarmentosa extract in a cyclophosphamide-induced immunosuppression mouse model, aiming to validate its traditional use and explore its potential as a novel immunotherapeutic adjunct. Swiss mice were divided into six groups, including controls and three L. sarmentosa treatment groups (36, 72, 108 mg/kg). Immunosuppression was induced by cyclophosphamide (200 mg/kg) on day 4 of a 7-day treatment. Key immune parameters evaluated included relative organ weights, leukocyte counts, delayed-type hypersensitivity (DTH) response, serum cytokine levels (IL-4, IL-6, IFN-γ, TNF-α, IgG), and lymphoid organ histopathology. L. sarmentosa extract significantly ameliorated cyclophosphamide-induced immunosuppression. Treatment, especially at 108 mg/kg/day, restored relative spleen weight and tended to enhance DTH reactions. The extract significantly elevated IL-4, IL-6, and IgG concentrations, modulating humoral and innate immune responses. Histopathological examination showed marked improvement in lymphoid organ damage, with increased lymphocyte density and restored tissue architecture in both spleen and thymus. While TNF-α and IFN-γ levels showed upward trends, these were not statistically significant. These findings suggest L. sarmentosa possesses potent immunomodulatory effects by protecting lymphoid organs, enhancing cytokine production, and supporting immune responses. Further studies are needed to elucidate its exact mechanisms and identify active compounds.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1. Nicholson LB. The immune system. Essays Biochem. 2016;60(3):275-301. https://doi.org/10.1042/EBC20160017
2. Alanazi HH, Elasbali AM, Alanazi MK, El Azab EF. Medicinal Herbs: Promising Immunomodulators for the Treatment of Infectious Diseases. Mol Basel Switz. 2023;28(24):8045. https://doi.org/10.3390/molecules28248045
3. Chaplin DD. Overview of the immune response. J Allergy Clin Immunol. 2010;125(2 Suppl 2):S3-23. https://doi.org/10.1016/j.jaci.2009.12.980
4. Morimoto Y, Routes JM. Immunodeficiency Overview. Prim Care Clin Off Pract. 2008;35(1):159-173. https://doi.org/10.1016/j.pop.2007.09.004
5. Rébé C, Ghiringhelli F. Cytotoxic effects of chemotherapy on cancer and immune cells: how can it be modulated to generate novel therapeutic strategies? Future Oncol Lond Engl. 2015;11(19):2645-2654. https://doi.org/10.2217/fon.15.198
6. Öhrmalm L, Smedman C, Wong M, Broliden K, Tolfvenstam T, Norbeck O. Decreased functional T lymphocyte-mediated cytokine responses in patients with chemotherapy-induced neutropenia. J Intern Med. 2013;274(4):363-370. https://doi.org/10.1111/joim.12100
7. Lagou MK, Anastasiadou DP, Karagiannis GS. A Proposed Link Between Acute Thymic Involution and Late Adverse Effects of Chemotherapy. Front Immunol. 2022;13:933547. https://doi.org/10.3389/fimmu.2022.933547
8. Ahlmann M, Hempel G. The effect of cyclophosphamide on the immune system: implications for clinical cancer therapy. Cancer Chemother Pharmacol. 2016;78(4):661-671. https://doi.org/10.1007/s00280-016-3152-1
9. Loeffler M, Krüger JA, Reisfeld RA. Immunostimulatory effects of low-dose cyclophosphamide are controlled by inducible nitric oxide synthase. Cancer Res. 2005;65(12):5027-5030. https://doi.org/10.1158/0008-5472.CAN-05-0646
10. Zhong Z, Vong CT, Chen F, Tan H, Zhang C, Wang N, Cui L, Wang Y, Feng Y. Immunomodulatory potential of natural products from herbal medicines as immune checkpoints inhibitors: Helping to fight against cancer via multiple targets. Med Res Rev. 2022;42(3):1246-1279. https://doi.org/10.1002/med.21876
11. Nikiema WA, Ouédraogo M, Ouédraogo WP, Fofana S, Ouédraogo BHA, Delma TE, Amadé B, Abdoulaye GM, Sawadogo AS, Ouédraogo R, Semde R. Systematic Review of Chemical Compounds with Immunomodulatory Action Isolated from African Medicinal Plants. Molecules. 2024;29(9):2010. https://doi.org/10.3390/molecules29092010
12. Shorinwa OA, Otu VS. Immunomodulatory Activity of the Aqueous Extract of the pith of Citrus limon L. (Rutaceae) Using Cyclophosphamide Induced Myelosuppression. Trop J Nat Prod Res. 2023;7(3):2660-2664 . http://www.doi.org/10.26538/tjnpr/v7i3.29.
13. Ezeagha CC, Ajaghaku DL, Ajaegbu EE, Ngwoke KG, Osadebe PO, Okoye FBC. Molucidine and Desmethylmolucidine as Immunostimulatory Lead Compounds of Morinda lucida (Rubiaceae). Trop J Nat Prod Res. 2021;5(5):977-982. http://www.doi.org/10.26538/tjnpr/v5i5.29
14. Marbun RAT, Rosidah, Yuandani, Sitorus P. Immunomodulatory effect of Artemisia vulgaris L. ethanol extract and its marker compound on antibody and cytokine release in non-immunosuppressed and immunosuppressed rats. Trop J Nat Prod Res. 2025;9(3):1228-1231. https://doi.org/10.26538/tjnpr/v9i3.44
15. Kan LLY, Chan BCL, Leung PC, Wong CK. Natural-Product-Derived Adjunctive Treatments to Conventional Therapy and Their Immunoregulatory Activities in Triple-Negative Breast Cancer. Molecules. 2023;28(15):5804. https://doi.org/10.3390/molecules28155804
16. Tran DQ, Pham AC, Nguyen TTT, Vo TC, Vu HD, Ho GT, Mohsin SM. Growth, Physiological, and Biochemical Responses of a Medicinal Plant Launaea sarmentosa to Salinity. Horticulturae. 2024;10(4):388. https://doi.org/10.3390/horticulturae10040388
17. Salih Y, Harisha CR, Shukla VJ, Acharya R. Pharmacognostical evaluation of Launaea sarmentosa (Willd.) schultz-bip.ex Kuntze root. Ayu. 2013;34(1):90-94. https://doi.org/10.4103/0974-8520.115439
18. Nguyen TT, Nguyen TTA, Nguyen TM, Nguyen THL, Nguyen TH, Vu TD, Tran HT, Nguyen TH, Nguyen MK, Do TH. Chemical constituents from the aerial parts of Launaea sarmentosa (Asteraceae). Biochem Syst Ecol. 2023;110:104706. https://doi.org/10.1016/j.bse.2023.104706
19. Pham TV, Hoang TX, Nguyen HN, Do BH, Vo HHN, Tran GB. Chemical Composition, Anti-bacterial Activity and Molecular Docking Studies of Essential Oil Isolated from Sa Sam Nam (Launaea sarmentosa). J Oleo Sci. 2024;73(5):787-799. https://doi.org/10.5650/jos.ess23254
20. Raju GS, RahmanMoghal MM, Hossain MS, Hassan MM, Billah MM, Ahamed SK, Rana SM. Assessment of pharmacological activities of two medicinal plant of Bangladesh: Launaea sarmentosa and Aegialitis rotundifolia Roxb in the management of pain, pyrexia and inflammation. Biol Res. 2014;47(1):55. https://doi.org/10.1186/0717-6287-47-55
21. Das S, Priyanka KR, Prabhu K, Vinayagam R, Rajaram R, Kang SG. Anticandidal Properties of Launaea sarmentosa among the Salt Marsh Plants Collected from Palk Bay and the Gulf of Mannar Coast, Southeastern India. Antibiotics. 2024;13(8):748. https://doi.org/10.3390/antibiotics13080748
22. Nguyen T, Hong T, Vo Thanh K, Y H, Pham DT, Giao D, Tran T, Kamei K. Anti-inflammatory Constituents Isolated From Launaea sarmentosa Against Infection by LPS-stimulated Macrophages. Rec Nat Prod. 2024;6:663-73 https://doi.org/10.25135/rnp.487.2410.3343
23. Shen L, Luo H, Fan L, Tian X, Tang A, Wu X, Dong K, Su Z. Potential Immunoregulatory Mechanism of Plant Saponins: A Review. Mol Basel Switz. 2023;29(1):113. https://doi.org/10.3390/molecules29010113
24. Oo AM, Nor MNM, Lwin OM, Simbak N, Mohd Adnan LH, Rao USM. Immunomodulatory effects of apigenin, luteolin, and quercetin through natural killer cell cytokine secretion. J Appl PharmSci.2022;12(09):121-26. https://doi.org/10.7324/JAPS.2022.120914
25. Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines. 2018;5(3):93. https://doi.org/10.3390/medicines5030093
26. Martínez G, Mijares MR, De Sanctis JB. Effects of Flavonoids and Its Derivatives on Immune Cell Responses. Recent Pat Inflamm Allergy Drug Discov. 2019;13(2):84-104. https://doi.org/10.2174/1872213X13666190426164124
27. Pham BQ, Nguyen NKT, Pham SQ, Nguyen CD, Trinh LV, Dinh HTT, Trinh QV, Pham VAT. Potential effects of Linh Loc Son hard capsule – a Vietnamese herbal combination in immunodeficiency induced by cyclophosphamide on mice. J Pharm Pharmacogn Res. 2024;12(5):892-899. https://doi.org/10.56499/jppres23.1751_12.5.892
28. Van Pham AT, Luong MH, Dinh HTT, Mai TP, Trinh QV, Luong LH. Immunostimulatory Effect of Moringa oleifera Extracts on Cyclophosphamide-induced Immunosuppressed Mice. J Herbs Spices Med Plants. 2021;27(4):377-385. https://doi.org/10.1080/10496475.2021.1934620
29. Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008:pdb.prot4986. https://doi.org/10.1101/pdb.prot4986
30. Zhong W, Huang H, Yang Z, Chang P. rhCNB Improves Cyclophosphamide-Induced Immunodeficiency in BALB/c Mice. Evid-Based Complement Altern Med ECAM. 2022;2022:4891399. https://doi.org/10.1155/2022/4891399
31. Khan S, Uddin M, Hridoy A, Islam K, Miah M. The Effect of Levamisole on Growth Performance, Humoral Immunity and Blood Biochemical Profile in Broiler Chickens. J Bangladesh Agric Univ. 2020;18. https://doi.org/10.5455/JBAU.13301
32. Pourahmad M, Soltani R, Noroozi MH, Khorvash F, Ataei B, Shams M, Nikokar F. Effectiveness of levamisole in the treatment of patients with severe COVID-19: a randomized controlled clinical trial. J Infect Dev Ctries. 2024;18(12.1):S275-S281. https://doi.org/10.3855/jidc.18659
33. Thapa P, Farber DL. The Role of the Thymus in the Immune Response. Thorac Surg Clin. 2019;29(2):123-131. https://doi.org/10.1016/j.thorsurg.2018.12.001
34. Xu J, Xu Z, Zhu S, Tovanich S, Wu B. Abstract C43: Cancer effects on the function of the host immune organs: Thymus and spleen. Cancer Prev Res (Phila Pa). 2013;6(11_Supplement):C43-C43. https://doi.org/10.1158/1940-6215.PREV-13-C43
35. Kolathingal-Thodika N, Usha PTA, Sujarani S, Suresh NN, Priya PM, Naseef PP, Kuruniyan MS, Ollakkode S, Elayadeth-Meethal M. A cyclophosphamide-induced immunosuppression Swiss Albino mouse model unveils a potential role for cow urine distillate as a feed additive. J Ayurveda Integr Med. 2023;14(5):100784. https://doi.org/10.1016/j.jaim.2023.100784
36. Dubyak GR. Chapter 21 - GPCRs in innate and adaptive immune responses. In: Jastrzebska B, Park PSH, editors. GPCRs: Academic Press; 2020. 429-461 p. https://doi.org/10.1016/B978-0-12-816228-6.00021-0
37. McDaniel MM, Meibers HE, Pasare C. Innate control of adaptive immunity and adaptive instruction of innate immunity: bi-directional flow of information. Curr Opin Immunol. 2021;73:25-33. https://doi.org/10.1016/j.coi.2021.07.013
38. Ganusov VV, Auerbach J. Mathematical modeling reveals kinetics of lymphocyte recirculation in the whole organism. PLoS Comput Biol. 2014;10(5):e1003586. https://doi.org/10.1371/journal.pcbi.1003586
39. Goldberg GL, Dudakov JA, Reiseger JJ, Seach N, Ueno T, Vlahos K, Hammett MV, Young LF, Heng TSP, Boyd RL, Chidgey AP. Sex Steroid Ablation Enhances Immune Reconstitution Following Cytotoxic Antineoplastic Therapy in Young Mice. J Immunol. 2010;184(11):6014-6024. https://doi.org/10.4049/jimmunol.0802445
40. Kalish RS, Askenase PW. Molecular mechanisms of CD8+ T cell–mediated delayed hypersensitivity: Implications for allergies, asthma, and autoimmunity. J Allergy Clin Immunol. 1999;103(2):192-199.https://doi.org/10.1016/S0091-6749(99)70489-6
41. Thorn M, Hudson AW, Kreeger J, Kawabe TT, Bowman CJ, Collinge M. Evaluation of a novel delayed-type hypersensitivity assay to Candida albicans in adult and neonatal rats. J Immunotoxicol.2015;12(4):350-360. https://doi.org/10.3109/1547691X.2014.980925
42. Ou JY, Liu FL, Chen CL, Fang MC, Huang CH. Immunomodulatory effects of Ulva-derived polysaccharides, oligosaccharides, and residues in a murine model of delayed-type hypersensitivity. Biosci Microbiota Food Health. 2024;43(2):128-134. https://doi.org/10.12938/bmfh.2023-065
43. Chongpison Y, Sriswasdi S, Buranapraditkun S, Thantiworasit P, Rerknimitr P, Mongkolpathumrat P, Chularojanamontri L, Srinoulprasert Y, Rerkpattanapipat T, Chanprapaph K, Disphanurat W, Chakkavittumrong P, Tovanabutra N, Srisuttiyakorn C, Sukasem C, Tuchinda P, Pongcharoen P, Klaewsongkram J. IFN-γ ELISpot-enabled machine learning for culprit drug identification in nonimmediate drug hypersensitivity. J Allergy Clin Immunol. 2024;153(1):193-202. https://doi.org/10.1016/j.jaci.2023.08.026
44. Jang D in, Lee AH, Shin HY, Song HR, Park JH, Kang TB, Lee SR, Yang SH. The Role of Tumor Necrosis Factor Alpha (TNF-α) in Autoimmune Disease and Current TNF-α Inhibitors in Therapeutics. Int J Mol Sci. 2021;22(5):2719. https://doi.org/10.3390/ijms22052719
45. Psarras A, Antanaviciute A, Alase A, Carr I, Wittmann M, Emery P, Tsokos G, Vital E. TNF-α Regulates Human Plasmacytoid Dendritic Cells by Suppressing IFN-α Production and Enhancing T Cell Activation. J Immunol. 2021;206(4):785-796. https://doi.org/10.4049/jimmunol.1901358
46. Silva-Filho JL, Caruso-Neves C, Pinheiro AAS. IL-4: an important cytokine in determining the fate of T cells. Biophys Rev. 2014;6(1):111-118. https://doi.org/10.1007/s12551-013-0133-z
47. Chakma CR, Good-Jacobson KL. Requirements of IL-4 during the Generation of B Cell Memory. J Immunol. 2023;210(12):1853-1860. https://doi.org/10.4049/jimmunol.2200922
48. Rath T, Billmeier U, Waldner MJ, Atreya R, Neurath MF. From physiology to disease and targeted therapy: interleukin-6 in inflammation and inflammation-associated carcinogenesis. Arch Toxicol. 2015;89(4):541-554. https://doi.org/10.1007/s00204-015-1461-5
49. Gabay C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8(2):S3. https://doi.org/10.1186/ar1917
50. Lin YL, Chen SH, Wang JY. Critical role of IL-6 in dendritic cell-induced allergic inflammation of asthma. J Mol Med. 2016;94(1):51-59. https://doi.org/10.1007/s00109-015-1325-8
51. Nauta J. Humoral and Cellular Immunity. In: Statistics in Clinical Vaccine Trials. Berlin Heidelberg: Springer; 2011. 13-17 p. https://doi.org/10.1007/978-3-642-14691-6_2
52. Manetti M, Pratesi S, Romano E, Rosa I, Martiradonna A, Bellando-Randone S, Guiducci S, Ibba-Manneschi L, Maggi E, Matucci-Cerinic M. FRI0362 Angiogenic t cell expansion correlates with severity of peripheral vascular damage in systemic sclerosis. Ann Rheum Dis. 2017;76:623. https://doi.org/10.1136/annrheumdis-2017-eular.3017
53. Xu YD, Cheng M, Shang PP, Yang YQ. Role of IL-6 in dendritic cell functions. J Leukoc Biol. 2022;111(3):695-709. https://doi.org/10.1002/JLB.3MR0621-616RR