Antimicrobial, Antioxidant and In Vivo Toxicity Studies of Triumfetta rhomboidea Jacq Leaf Extract in Male Wistar Rats
Main Article Content
Abstract
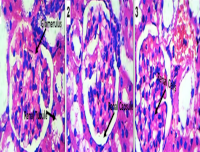
Although Triumfetta rhomboidea (TR) has found its place in folklore medicinal applications, the scientific basis of these applications is poorly understood. This study examined the antioxidant and antibacterial properties of TR and its potential alteration of the renal and liver functions. The chemical constituents and antioxidant capacity of the ethanol extract of TR leaves were determined using FTIR, GC-MS and standard methods, and the agar well diffusion method was used to measure the antimicrobial activity. For the toxicity study, fifteen rats were divided into three groups: A – C; control, 200 mg/kg and 300 mg/kg ethanol TR extract per body weight administered orally for fourteen days. Biochemical assays were performed on the plasma, liver and kidney homogenates, and histo-pathological changes were examined in the tissues. Results showed that TR leaves contain total flavonoids (0.1316 ± 0.0001 mg/ GAE/100g) and total phenols (0.13 ± 0.0002 mg GAE/100g). GC-MS analysis of the leaf extract showed the presence of 44 major compounds. The extract demonstrated significant inhibitory activity against Bacillus cereus, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Salmonella typhimurium. Biochemical assay revealed no significant effect on the liver and renal function changes compared to the control group, except for the critical (p< 0.05) increase in Na. The antioxidant parameters in both the kidney and liver homogenates of TR-treated rats were not significantly altered except for reduced SOD levels at 200 mg/kg. Histopathological assay revealed relative degenerative changes in the two organs. TR leaves have antimicrobial and antioxidant properties, but could be toxic to the liver and kidneys at high doses.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.Thawkar BS, Kale M, Oswal M, Maniyar K, Kadam K, Kamat S. To study the antioxidant activity of 70% methanolic extract of Triumfetta rhomboidea by DPPH radical scavenging.
2.European J Biomed Pharm Sci. 2016; 3(11): 200–203. DOI:10.5958/0974-360X.2016. 00044.5
3.Kendre N, Wakte P. Triumfetta rhomboidea: a review on its phytochemical and pharmacological profile. Int J Pharm Sci Res. 2022; 13(9): 3458-3464. DOI:10.13040/IJPSR. 0975-8232.13(9).3458-64
4.Momo A, Obianime AW, Uche FI, Berebon DP, Odimegwu DC. Aqueous Extract of Triumfetta rhomboidea Jacq Leaves Modulates Hormones and Upregulates Male Fertility Function in Guinea Pig. Biomed Pharmacol J. 2020; 13(3). DOI:10.13005/bpj/1980
5.Chandimali N, Bak SG, Park EH, Lim HJ, Won YS, Kim EK, Park SI, Lee SJ. Free radicals and their impact on health and antioxidant defences: a review. Cell Death Discov. 2025; 11(1):19. DOI: 10.1038/s41420-024-02278-8.
6.García-Sánchez A, Miranda-Díaz AG, Cardona-Muñoz EG. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxid Med Cell Longev. 2020; 2020:2082145. DOI:10.1155/2020/20821 45
6.Oguntibeju OO. Type 2 diabetes mellitus, oxidative stress and inflammation: examining the links. Int J Physiol Pathophysiol Pharmacol. 2019; 11(3): 45–63. PMCID:PMC3040941 PMID: 21383873
7.Mariani E, Polidori MC, Cherubini A, Mecocci P. Oxidative stress in brain ageing, neurodegenerative and vascular diseases: an overview. J Chromatogr B. 2005; 827(1): 65–75. DOI.10.1016/j.jchromb.2005.04.023.
8.Nii-Trebi NI. Emerging and Neglected Infectious Diseases: Insights, Advances, and Challenges. Biomed Res Int. 2017; 1–15. DOI: 10.1155/2017/5245021.
9.AlSheikh HMA, Sultan I, Kumar V, Rather IA, Al-Sheikh H, Tasleem Jan A, Haq QMR. Plant-Based Phytochemicals as Possible Alternative to Antibiotics in Combating Bacterial Drug Resistance. Antibiotics (Basel). 2020; 9(8):480. DOI:10.3390/antibiotics9080480.
10.Abdallah EM, Alhatlani BY, de Paula Menezes R, Martins CHG. Back to Nature: Medicinal Plants as Promising Sources for Antibacterial Drugs in the Post-Antibiotic Era. Plants (Basel). 2023; 12(17): 3077. DOI:10.3390/plants12173077.
11.Kumar A, Nirmal P, Kumar M, Jose A, Tomer V, Oz E, Proestos C, Zeng M, Elobeid T, Sneha K, Oz F. Major Phytochemicals: Recent Advances in Health Benefits and Extraction Method. Molecules. 2023; 28(2): 887. DOI: 10.3390/molecules28020887.
12. Nollet LM, Gutierrez-Uribe JA. Phenolic Compounds in Food: Characterisation and Analysis 1st Ed., 167 (CRC Press, 2018). DOI:10.1201/9781315120157.
13.Greenhough S, Hay DC. Stem cell-based toxicity screening. Pharm Med. 2012; 2: 85-89. DOI:10.1007/BF03256896
14.Akintimehin, ES, Iyere OO. Acute and Sub-Chronic Toxicity Studies of Aqueous and Ethanol Extracts of Triumfetta 14.Rhomboidea (Tiliaceae) Leaf in Healthy Albino Rats. Vegetos. 2023; 37 (6): 2532– 2541. DOI:10.1007/s42535-023-00762-7
15. Olaniyi MB, Lawal IO, Olaniyi AA. 'Proximate, phytochemical screening and mineral analysis of Crescentia cujete L. leaves', J Med Plant Econ Dev. 2018; 2: a28. DOI:10.4102/ jomped.v2i1.28
16. Singleton VL, Rossi Jr JA. Colourimetry of total phenolics with phosphomolybdic acid. Am J Enol Vitic. 1965; 16: 144-158. DOI:10.5344/ajev.1965.16.3.144
17.Marinova D, Ribarova F, Atanassova M. Total Phenolics and Total Flavonoids in Bulgarian Fruits and Vegetables. J Uni Chem Technol Metal. 2005; 40: 255-260.
18.Alhakmani F, Kumar S, Khan SA. Estimation of total phenolic content, in-vitro antioxidant and anti-inflammatory activity of flowers of Moringa oleifera, Asian Pac J Trop Biomed. 2013; 3(8): 623–627. DOI:10.1016/S2221-1691(13)60126-4
19.Marcocci L, Maguire JJ, Droy-Lefaix MT, Packer L. The nitric oxide-scavenging properties of Ginkgo biloba extract EGb 761. Biochem Biophys Res Commun. 1994; 201(2):748-755. DOI: 10.1006/bbrc.1994.1764.
20.Gulcin I, Tel AH, Kirecci E. Antioxidant, Antimicrobial, Antifungal, and Antiradical Activities of Cyclotrichium niveum (BOISS.). Int. J. Food Prop. 2008;11(2):450-471. DOI: 10.1080/10942910701567364.
21.Prieto P, Pineda M, Aguila M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal Biochem. 1999; 269(2):337-41. DOI: 10.1006/abio. 1999.4019.
22.NIST Chemistry WebBook NIST Standard Reference Database Number 69 (2022).
23.Mostafa AA, Al-Askar AA, Almaary KS, Dawoud TM, Sholkamy EN, Bakri MM. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J Biol Sci. 2018; 25: 361–366. DOI:10.1016/j.sjbs.2017.02.004.
24.Jollow DJ, Mitchell JR, Zampaglione N, Gillette JR. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3, 4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974; 11(3):151-169. DOI: 10.1159/000136485.
25.Rotruck, J.T., Pope, A.L., Ganther, H.E., Swanson, A.B., Hafeman, D.G., Hoekstra, W.G. Selenium: Biochemical role as a component of glutathione peroxidase. Science. 1973; 179(4073):588-590. DOI: 10.1126/science.179.4073.588.
26. Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974; 249(22):7130-7139. DOI:10.1016/S0021-9258(19)42083-8.
27. Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972; 247(10):3170-3175. DOI:10.1016/S0021-9258(19)45228-9.
28.Claiborne A. Catalase activity. In: Greenwald, R.A., Ed., CRC Handbook of Methods for Oxygen Radical Research, CRC Press, Boca Raton, 1985; 283-284.
29.Varshney R, Kale RK. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol. 1990; 58(5):733-43. DOI: 10.1080/09553009014552121.
30.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein Measurement with the Folin Phenol Reagent. J. Biol. Chem. 1951;193(1):265-275. DOI:10.1016/S0021-9258(19)52451-6
31.Akintimehin, E., Onoagbe, I., Abu, O. Proximate Composition, Qualitative Phytochemical Screening and In-Vitro Antioxidant Potential of Triumfetta rhomboidea Leaves Extracts. Trends Sci. 2022; 19(24), 3033. DOI:10.48048/tis.2022.3033.
32.Joshi RK. GC-MS Analysis of Volatile Organic Constituents of Traditionally Used Medicinal Plants from the Western Ghats of India: Blumea lanceolaria (Roxb.) Druce, Heliotropium indicum L. and Triumfetta rhomboidea Jacq. J Mex Chem Soc. 2020, 64(2): 74 – 82. DOI:10.29356/jmcs.v64i2.1093.
33.Górniak I, Bartoszewski R, Króliczewski J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem Rev. 2018; 18: 241-272. DOI: 10.1007/s11101-018-9591-z
34.Coates J. Interpretation of Infrared Spectra: A Practical Approach. In: Meyers, R.A., Ed., Encyclopedia of Analytical Chemistry, John Wiley & Sons Ltd., Chichester. 2000; 10881-10882. DOI: 10.1002/9780470027318.a5606.
35.Ogah C, Sule J, Aluko V, Gyebi G, Anyanwu G. Piliostigma thonningii (Schum.) Milne-Redh: GC-MS Profiling, In vitro, In vivo Antioxidant Potential and Toxicological Assessment in Female Wistar Rats. Trop J Nat Prod Res. 2025; 9(7): 3297 – 3304. DOI:10.26538/tjnpr/ v9i7.58.
36.Asbaghi O, Sadeghian M, Nazarian B, Sarreshtedari M, Mozaffari-Khosravi H, Maleki V, Alizadeh M, Shokri A, Sadeghi O. The effect of vitamin E supplementation on selected inflammatory biomarkers in adults: a systematic review and meta-analysis of randomised clinical trials. Sci Rep 2020; 10(1):17234. DOI: 10.1038/s41598-020-73741-6.
37.Olukanni OD, Abiola T, Olukanni AT, Ojo AV. Chemical Composition, In Silico and In Vitro Antimutagenic Activities of Ethanolic and Aqueous Extracts of Tigernut (Cyperus esculentus). Prev Nutr Food Sci. 2022; 27(2):198-211. DOI: 10.3746/pnf.2022.27.2.198.
38.Odeghe BO, Onobrudu AD, Orororo CO, Ikoya S, Apitikori-Owumi EJ, Egbune OE, Nwogueze CB, Ojugbeli TE, Agboola EO, Awhin PE, EkakitieI L, Oviri OM, Ofoke HI, Cyril C. Dunkwu, Enyi CK, Derhie AV, Isaac OP,Mordi CJ ,Adebayo O. Adegoke OA. Phytochemical Profiling, GC-MS Analysis, and Antioxidant Activity of Polar Solvent Extracts of Conoclinium coelestinum Leaves. Trop J Nat Prod Res. 2025; 9(7): 3214 –3224 DOI:10.26538/tjnpr/v9i7.
39.Zai MJ, Cheesman MJ, Cock IE. Phytochemical Evaluation of Terminalia canescens DC. Radlk. Extracts with Antibacterial and Antibiotic Potentiation Activities against Selected β-Lactam Drug-Resistant Bacteria. Molecules. 2024; 29(6):1385. DOI:10.3390/molecules 29061385.
40.Es-Sai B, Wahnou H, Benayad S, Rabbaa S, Laaziouez Y, El Kebbaj R, Limami Y, Duval RE. Gamma-Tocopherol: A Comprehensive Review of Its Antioxidant, Anti-Inflammatory, and Anticancer Properties. Molecules. 2025; 30(3):653. DOI: 10.3390/molecules30030653.
41.Korbecki J, Bajdak-Rusinek K. The effect of palmitic acid on inflammatory response in macrophages: an overview of molecular mechanisms. Inflamm. Res. 2019; 68(11):915-932. DOI: 10.1007/s00011-019-01273-5.
42.Mi H, Wang D, Xue Y, Zhang Z, Niu J, Hong Y, Drlica K, Zhao, X. Dimethyl Sulfoxide Protects Escherichia coli from Rapid Antimicrobial-Mediated Killing. Antimicrob Agents Chemother. 2016; 60(8):5054-5058. DOI:10.1128 /AAC.03003-15.
43.Otuechere CA, Adewuyi A, Ekozi A, Feng X, Cabrerizo FM, Green Synthesised Zinc Oxide Nanoparticles Elicited a Prominent Suppression of Oxidative and Inflammatory Distortions in Rats Exposed to Carbon Tetrachloride. Biointerface Res Appl Chem. 2022; 12: 5444 – 5457. DOI:10.33263/BRIAC124.54445457.
44.Olukanni AT, Omotosho D, Olalekan DT, Durugbo E, Adewumi AT, Olukanni OD, Mosebi S. Hepatoprotective and Nephroprotective Effects of Leea guineensis Leaf Extract Against Paracetamol-Induced Toxicity: Combined Mouse 44.Model-Integrated in Silico Evidence. Int J Mol Sci 2025; 26(13):6142. DOI: 10.3390/ijms26136142.
45.Alkandahri MY, Sadino A, Abriyani E, Hermanto F, Oktoba Z, Sayoeti MFW, Sangging PRA, Wardani D, Hasan N, Sari SW, Safitri NA, Ikhtianingsih W, Safitri S. Evaluation of hepatoprotective and nephroprotective activities of Castanopsis costata extract in rats. Biomed Rep. 2024; 22(2):24. DOI: 10.3892/br.2024.1902.
46.Moraes Carlesso R, Cappellari YLR, Boeff DD, da Costa Pereira A, Schmitt Rusch E, de Souza Claudino T, Ritter MR, Konrath EL. Nephroprotective Plant Species Used in Brazilian Traditional Medicine for Renal Diseases: Ethnomedical, Pharmacological, and Chemical Insights. Plants (Basel). 2025; 14(5):648. DOI: 10.3390/plants14050648.
47.Chaudhary P, Janmeda P, Docea AO, Yeskaliyeva B, Abdull Razis AF, Modu B, Calina D and Sharifi-Rad J. Oxidative stress, free radicals and antioxidants: potential crosstalk in the pathophysiology of human diseases. Front Chem. 2023; 11:1158198. DOI:10.3389/fchem. 2023.1158198.
48.Klomjit N, Ungprasert P. Acute kidney injury associated with non-steroidal anti-inflammatory drugs. Eur J Intern Med. 2022; 101:21-28. DOI: 10.1016/j.ejim.2022.05.003.
49.Asatiani N, Sapojnikova N, Kartvelishvili T, Asanishvili L, Sichinava N, Chikovani Z. Blood Catalase, Superoxide Dismutase, and Glutathione Peroxidase Activities in Alcohol- and Opioid-Addicted Patients. Medicina (Kaunas). 2025; 61(2):204. DOI: 10.3390/medicina 61020204.
50.Abdi M, Karimzadeh H, Jourabchi A, Seghinsara AM, Khodaie L. Protective effects of Tribulus Terrestris extract on cisplatin-induced ovarian damage: Antioxidants and anti-inflammatory insights. Hum Exp Toxicol. 2025; 44: 1-9. DOI: 10.1177/096032712513534 92.