Toxicological Assessment of Methanol Root Extract of Newbouldia laevis in Rats After Therapeutic Evaluation Against Plasmodium berghei in Mice
Main Article Content
Abstract
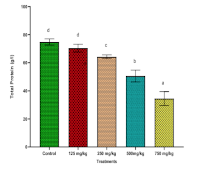
This study evaluated the toxicity of methanol extracts of Newbouldia laevis in rats. Phytochemical screening was done using standard methods. Twenty five Wistar rats were divided into five groups of five rats each. The control group received 0.9% normal saline. The remaining groups were administered once daily for four days; 125, 250, 500, and 750 mg/kg body weight of the extract and afterwards monitored for 14 days. At the end, blood and organ samples were collected for biochemical, hematological and histopathological evaluation. Appreciable amount of flavonoids, alkaloids, saponins, tannins, oxalate and phytate were present. Significantly increased ALT activity of 65.28±1.55 U/L and 71.05±1.28 U/L were recorded at higher extract treatment doses of 500 and 750 mg/kg respectively compared to 34.71±1.99 U/L for the Control, just as AST and ALP presented similar trend of increased activities. Concentrations of total protein dropped from 74.74±2.25 g/dL (control) to 34.49±4.97 g/dL, while Urea (11.91±0.20 mg/dL) and creatinine (108.88±5.31 mg/dL) increased significantly compared to 5.36±0.41 mg/dL and 68.23±3.33 mg/dL recorded for controls respectively, indicating dose-dependent liver and kidney dysfunction. Other liver, kidney and haematological parameters varied significantly (p ≤ 0.05) compared to the Control. Organ histology revealed pathological abnormalities in liver and kidney of treated rats at higher doses. This evaluation presented significant dose-dependent alterations in biochemical, haematological, histological parameters, indicating potential organ-specific toxicity at higher doses. These findings have shown N. laevis methanol root extract as a potential alternative treatment for malaria and a source for drug development having shown potent antiplasmodial properties, however, proper dose regulation is needed in the use.
Downloads
Article Details
Section

This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
How to Cite
References
1.World Health Organization. World Malaria Day 2025. Geneva: World Health Organization. https://www.who.int/campaigns/world-malaria-day/2025
2.Fikadu M, Ashenafi E. Malaria: An overview. Infect Drug Resist. 2023;16:3339–47. https://doi.org/10.2147/IDR.S405668
3.Li S, Odedina S, Agwai I, Ojengbede O, Huo D, Olopade OI. Traditional medicine usage among adult women in Ibadan, Nigeria: A cross-sectional study. BMC Complement Med Ther. 2020;20(1):1–7. doi:10.1186/s12906-020-02881-z
4.Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C. Averting a malaria disaster: Will insecticide resistance derail malaria control? Lancet. 2016;387(10036):1785–8.
5.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med. 2018;379(10):983–93.
6.Plowe CV. Malaria chemoprevention and drug resistance: A review of the literature and policy implications. Malar J. 2022;21:104. https://doi.org/10.1186/s12936-022-04115-8
7.Willcox ML, Graz B, Falquet J, Diakite C. Artemisia annua as a malaria treatment: A review. J Altern Complement Med. 2011;17(10):1015–22.
8.Irungu B, Okari E, Nyangi M, Njeru S, Koech L. Potential of medicinal plants as antimalarial agents: A review of work done at Kenya Medical Research Institute. Front Pharmacol. 2023;14:1268924. https://doi.org/10.3389/fphar.2023.1268924
9.Ene AC, Egbosi NC, Obika CJ, Ibegbulem CO, Ujowundu CO, Alisi CS. In vivo antiplasmodial activity of ethanol and aqueous extracts of Uvaria chamae and Phyllantus amarus plants. FUTO J Ser (FUTOJNLS). 2016;2(2):83–97. Available from: http://www.futojnls.org
10.Ene AC, Ezeji-Chigbu NGN, Emejulu AA, Ene CU, Okwu GN, Ujowundu CO. Antiplasmodial and antioxidant evaluation of the methanol and aqueous extracts of Sarcocephalus latifolius. SciFed J Anal Biochem. 2018;1(1).
11.Habibi P, Shi Y, Grossi-de-Sá MF, Khan I. Plants as sources of natural and recombinant antimalaria agents. Mol Biotechnol. 2022;64(11):1177–97. https://doi.org/10.1007/s12033-022-00499-9
12.Federal Ministry of Health. National Malaria Strategic Plan 2014–2020. Abuja: Federal Ministry of Health; 2016.
13.Ujowundu CO, Morah AC, Ujowundu FN, Onyeocha IO, Igwe KO, Asiwe ES, Kalu JO, Onwuliri VA. Biocidal evaluation of ethanol leaf extract of Jatropha tanjorensis by inhibition of dehydrogenase activity of Staphylococcus aureus and Candida albicans. Trop J Nat Prod Res. 2022;6(6):951–6. https://doi.org/10.26538/tjnpr/v6i6.22
14.Ujowundu FN, Kalu JO, Ujowundu CO, Onyeocha IO, Onuoha CH, Ibeh RC, Obasi UK, Ntaji OE, Chigbu IF, Ezirim CY. Investigating the effect of flavonoid, saponin, alkaloids and tannins extracted from Combretum dolichopentalum Diels in CCl₄-induced hepatotoxicity. Trop J Nat Prod Res. 2022;6(8):1255–61. https://doi.org/10.26538/tjnpr/v6i8.16
15.Ajaiyeoba EO, Onocha PA, Nwozo SO. Antiplasmodial and anti-inflammatory activities of Newbouldia laevis leaf extract. J Ethnopharmacol. 2006;107(3):451–6.
16.Aderinola AA, Ejiofor JI, Chindo AB. Studies on the toxicological properties of ethanol stem-bark extract of Newbouldia laevis (P. Beauv) Seem in rats. Trop J Nat Prod Res. 2023;7(3):2665–73.
17.Buss AD, Butler MS. Natural product chemistry for drug discovery. Cambridge: Royal Society of Chemistry; 2010. p. 153.
18.Van Vuuren SF, Motlhallego KE, Netshia V. Traditionally used polyherbals in a southern African therapeutic context. J Ethnopharmacol. 2022;288:114977.
19.Boham BA, Kocipai-Abyazan R. Flavonoids and condensed tannins from leaves of Hawaiian vaccinium vaticulatum and V. calycinium. Pac Sci. 1974;48:458–63.
20.Harborne JB. Phytochemical methods: A guide to modern techniques of plant analysis. London: Chapman and Hall; 1973.
21.Obadoni PO, Ochuko MC. Practical methods of determining various components from plant extract. Adv Environ Med Biol. 2001;102:341–98.
22.Trease GE, Evans WC. Trease and Evans’ pharmacognosy. 13th ed. London: Bailliere Tindall; 1989.
23.Sofowora A. Medicinal plants and traditional medicine in Africa. 2nd ed. New York: John Wiley and Sons Ltd; 1993. p. 256.
24.Reitman S, Frankel S. A colorimetric method of determination of serum glutamic oxaloacetic and glutamic pyruvic transaminase. Am J Pathol. 1957;28:56–62.
25.Roy AV. Rapid method for determining alkaline phosphatase activity in serum with thymolphthalein monophosphate. Clin Chem. 1970;21(5):156–63.
26.Tietz NW. Clinical guide to laboratory tests. 3rd ed. Philadelphia: W.B. Saunders Company; 1995. p. 518–9.
27.Doumas BT, Watson WA, Biggs HG. Albumin standards and the measurement of serum albumin with bromocresol green. Clin Chim Acta. 1971;31:87–97.
28.Chaney AL, Marbach EP. Modified reagents for determination of urea and ammonia. Clin Chem. 1962;8:130–2.
29.Tietz NW. Fundamentals of clinical chemistry. 3rd ed. Philadelphia: W.B. Saunders Company; 1976.
30.Henry RJ. Clinical chemistry: principles and techniques. 2nd ed. Hagerstown: Harper and Row; 1974. p. 42–712. https://doi.org/10.3390/nu10111618
31.Tietz NW. Fundamentals of clinical chemistry. 3rd ed. Philadelphia: W.B. Saunders Company; 1987. p. 470–560.
32.Dacie JV, Lewis SM. Dacie and Lewis Practical Haematology. 11th ed.
33.Oduola T, Adeniyi FAA, Ogunyemi EO, Bello IS, Idowu TO. Toxicity studies on an unripe Carica papaya aqueous extract: Biochemical and haematological effects in Wistar albino rats. J Med Plants Res. 2006;4(12):1144–9.
34.Sysmex Corporation. KX-21N Automated Hematology Analyzer: Operator's Manual. Kobe, Japan: Sysmex Corporation; 1999. Available from: https://www.frankshospitalworkshop.com/equipment/documents/automated_analyzer/user_manuals/Sysmex%20KX-21%20Hematology%20Analyzer%20-%20Instruction%20manual.pdf
35.Okoro I. Manual of practical histology. 2nd ed. Owerri, Imo State: Peace Publishers; 2002.
36.World Health Organization. WHO guidelines on safety monitoring of herbal medicines in pharmacovigilance systems. Geneva: World Health Organization; 2018. Available from: https://apps.who.int/iris/handle/10665/43811
37.Chen X, Zhu Y, Zhang L, Wang J, Zhao Y, Xiao X. Pyrrolizidine alkaloid-induced hepatotoxicity associated with formation of reactive metabolite-derived pyrrole-protein adducts. Toxicol Lett. 2021;350:1–11.
38.Ujowundu CO, Nwokedinobi N, Kalu FN, Nwaoguikpe RN, Okechukwu RI. Chemoprotective potentials of Ocimum gratissimum in diesel petroleum-induced hepatotoxicity in albino Wistar rats. J Appl Pharm Sci. 2011;1(10):56–61.
39.Ujowundu CO, Okoye HN, Nwaoguikpe RN, Belonwu DC, Igwe KO, Ujowundu FN. Hepatoprotective effects of crude extracts of tomato and onion in rats exposed to locally processed beef. Int J Biochem Res Rev. 2014;4(2):193–203. https://doi.org/10.9734/IJBCRR/2014/7353
40.Murtala AA, Akindele AJ, Oreagba IA. Ninety-day toxicological assessment of preparation of the medicinal 40.plant Newbouldia laevis (P. Beauv.) Seem. (Bignoniaceae) in rats. Nat Prod Commun. 2024;19(6):1–10.
41.Tafere GG, Tuem KB, Gebre AK, Balasubramaniam R. In vitro antioxidant and in vivo hepatoprotective activities of root bark extract and solvent fractions of Croton macrostachyus Hochst. Ex Del. (Euphorbiaceae) on paracetamol-induced liver damage in mice. J Exp Pharmacol. 2020;12:301–11. https://doi.org/10.2147/JEP.S259081
42.Sun L, Yin H, Liu M, Xu G, Zhou X, Ge P, et al. Impaired albumin function: a novel potential indicator for liver function damage? Ann Med. 2019;51(7–8):333–44. https://doi.org/10.1080/07853890.2019.1693056
43.Lala V, Zubair M, Minter DA. Liver Function Tests. [Updated 2023 Jul 30]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025–. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482489/
44.Rehman MHU, Saleem U, Ahmad B, Rashid M. Phytochemical and toxicological evaluation of Zephyranthes citrina. Front Pharmacol. 2022;13:1007310. https://doi.org/10.3389/fphar.2022.1007310
45.Kang L, Li D, Jiang X, Zhang H, Liu Y, Zhu Y. Hepatotoxicity of the major anthraquinones derived from Polygoni multiflori radix based on bile-acid homeostasis. Front Pharmacol. 2022;13:878817. https://doi.org/10.3389/fphar.2022.878817
46.Xiang Z, Chen X, Luo Y, Wu L, Zhang L, Xiao X. Prognostic factors for pyrrolizidine alkaloid-induced hepatic sinusoidal-obstruction syndrome: A multicentre study in China. Hepatol Int. 2021;15:1013–23.
47.Ujowundu CO, Ajaegbo EO, Ujowundu FN, Okorie AA, Onyemuche SO, Nwajagu AI, Njoku MO. Chemoprotective potentials of selected dietary supplements in glyphosate-based herbicide-induced nephrotoxicity and dyslipidemia inalbino Wistar rats. Asian J Biol Sci. 2019;12:320–7. https://doi.org/10.3923/ajbs.2019.320.327
48.Emeribe AU, Anyanwu SO, Isong IK, Bassey UR, Inyang IJ, Ibeneme EO, Asemota EA, Okhormhe Z, Icha B, Abdullahi IN. Phytochemical analysis and toxicological evaluation of the ethanolic Leaves extract of Hypoestes rosea on the morphology and biochemical indices of the Kidneys of albino Wistar Rats. Saudi J Biol Sci. 2021;28(12):6748-6755. doi: 10.1016/j.sjbs.2021.07.045.
49.Obidah HA, Umaru HA, Barau NS. Effect of Vitellaria paradoxa (Shea butter) rich diet on gentamicin-induced nephrotoxicity in white Wistar rats. J Pre Clin Clin Res. 2022;16(1):1–5. https://doi.org/10.26444/jpccr/146923
50.Eneh GDO, Okon GG, Okon JE, Essien NB. Phytochemical studies and antidiabetic activities of Newbouldia laevis (P. Beauv) ethanolic leaves extracts in alloxan-induced diabetic rats. Int J Sci Res Chem. 2018;3(5):52–61.
51.Rui Y, Li S, Luan F, Li D, Liu R, Zeng N. Several alkaloids in Chinese herbal medicine exert protection in acute kidney injury: Focus on mechanism and target analysis. Oxid Med Cell Longev. 2022;2022:2427802. https://doi.org/10.1155/2022/2427802
52.Ushie OA, Longbap BD, Ugwuja DI, Iyen SI, Azuaga TI, Uba M. Preliminary phytochemical screening and proximate analyses of leaf extracts of Newbouldia laevis (Boundary tree). Dutse J Pure Appl Sci. 2021;7(3b):191–8. https://doi.org/10.4314/dujopas.v7i3b.21
53.Eluu SC, Oko AO, Eluu K, Okoye CS, Onyekwere UU, Omoniyi OA. Impact of Newbouldia laevis root extract on hematological parameters in rats: A comprehensive study on dosage-dependent effects and long-term dynamics. Niger Agric J. 2023;54(2):123–30.
54.Ene AC. Acute toxicity of aqueous leaf extract of Newbouldia laevis in Swiss albino mice. J Pharmacol Toxicol. 2022;17(1):1–8.
55.Abubakar Z, Dabo NT. Erythrocytic, enzymatic, and histological markers of oxidative stress in subacute and chronic stage infections in Wistar rats (Rattus norvegicus) infected with Trypanosoma brucei brucei. Dis Markers. 2023;2023:3590893. https://doi.org/10.1155/2023/3590893
56.Saleem N, Lashari MH, Ahmad HI, Tahreem S, Almutairi MH, Ahmed S. Hematological changes in the blood of experimental male and female albino rats on exposure to pesticide, dimethoate. PLoS One. 2025;20(5):e0321848. https://doi.org/10.1371/journal.pone.0321848
57.Baumann E, Stoya G, Völkner A, Richter W, Lemke C, Linss W. Hemolysis of human erythrocytes with saponin affects the membrane structure. Acta Histochem. 2000;102(1):21–35. https://doi.org/10.1078/0065-1281-00534
58.Orrico F, Laurance S, Lopez AC, Lefevre S, Thomson L, Möller MN, et al. Oxidative stress in healthy and pathological red blood cells. Biomolecules. 2023;13(8):1262. https://doi.org/10.3390/biom13081262
59.Guyton AC, Hall JE. Textbook of medical physiology. 12th ed. Philadelphia: Elsevier Saunders; 2011.
60.Park K. The role of dietary phytochemicals: Evidence from epidemiological studies. Nutrients. 2023;15(6):1371. https://doi.org/10.3390/nu15061371
61.Agbafor KN, Nwachukwu N. Phytochemical analysis and antioxidant property of leaf extracts of Vitex doniana and Mucuna pruriens. Biochem Res Int. 2011;2011:459839. https://doi.org/10.1155/2011/459839
62.Sharma B, John S. Hepatic cirrhosis. [Updated 2022 Oct 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025–. Available from: https://www.ncbi.nlm.nih.gov/books/NBK482419/
63.Lu Y, Tang W, Zhang H, Liu J, Zhong S. Effect of hepatocyte damage in hepatic fibrogenesis of patients infected with Schistosoma japonicum. Infect Immun. 2024;92(6):e0002624. https://doi.org/10.1128/iai.00026-24
64.Kew MC. Serum aminotransferase concentration as evidence of hepatocellular damage. Lancet. 2000;355(9204):591–2. https://doi.org/10.1016/S0140-6736(99)00219-6
65.Pessayre D, Fromenty B, Berson A, Robin MA, Lettéron P, Moreau R, et al. Central role of mitochondria in drug-induced liver injury. Drug Metab Rev. 2012;44(1):34–87. https://doi.org/10.3109/03602532.2011.604086
66.Pessayre D, Mansouri A, Berson A, Fromenty B. Mitochondrial involvement in drug-induced liver injury. Handb Exp Pharmacol. 2010;(196):311–65. https://doi.org/10.1007/978-3-642-00663-0_11
67.Jaeschke H, McGill MR, Ramachandran A. Oxidant stress, mitochondria, and cell death mechanisms in drug-induced liver injury: lessons learned from acetaminophen hepatotoxicity. Drug Metab Rev. 2012;44(1):88–106. https://doi.org/10.3109/03602532.2011.602688
68.Yan M, Huo Y, Yin S, Hu H. Mechanisms of acetaminophen-induced liver injury and its implications for therapeutic interventions. Redox Biol. 2018;17:274–83. https://doi.org/10.1016/j.redox.2018.04.019
69.Rashed K. Phytochemical and biological effects of Newbouldia laevis: A review. Plantae Sci. 2021;4(5):208–13. https://doi.org/10.32439/ps.v4i5.208-213
70.Yahfoufi N, Alsadi N, Jambi M, Matar C. The immunomodulatory and anti-inflammatory role of polyphenols. Nutrients. 2018;10(11):1618. https://doi.org/10.3390/nu10111618
71.Sahu SC, Long M, Walton JC. Saponins and their toxicological mechanisms. Toxicol Rep. 2022;9:857–64.